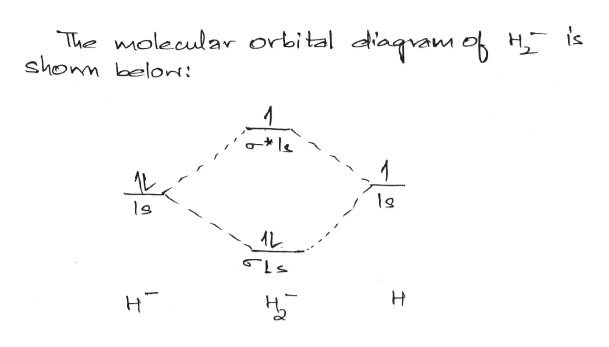
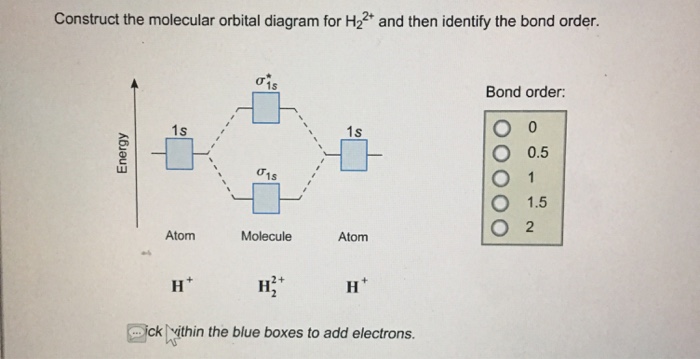
38 molecular orbital diagram for h2 2-
PDF Chapter 5 | 5.2.2 Orbital Mixing Molecular orbital theory uses group theory to describe the bonding in molecules; it comple-ments and extends the introductory bonding models in Chapter 3 . In molecular orbital theory the symmetry properties and relative energies of atomic orbitals determine how these orbitals interact to form... Molecular Orbital Theory - Chemistry | Socratic Molecular orbital theory is a method for determining molecular structure. It describes electrons as moving under the influence of the nucleus and not assigned to specific bonds. How do you draw an atomic level diagram for 2S+3O2=2SO3 ?
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.

Molecular orbital diagram for h2 2-
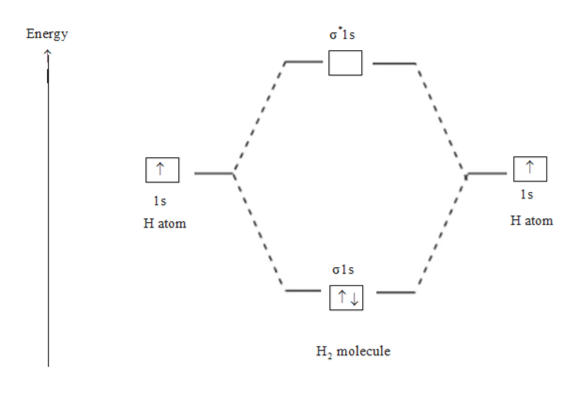
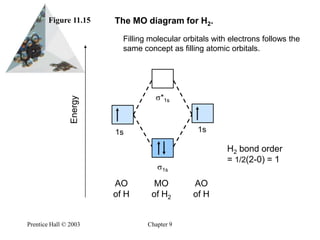
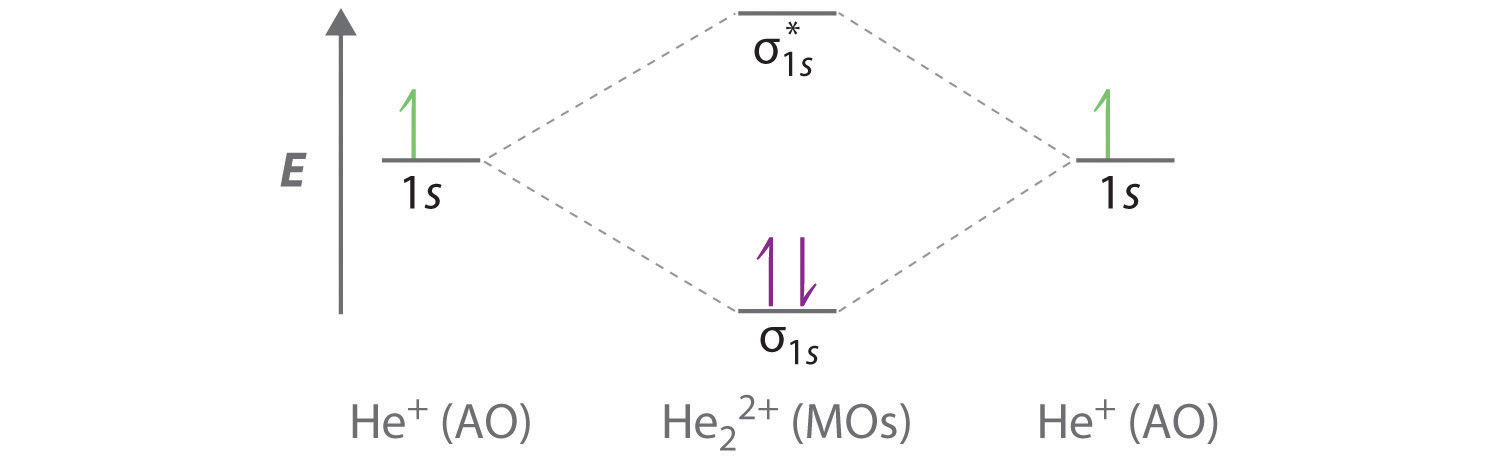
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Figure 8.35 The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. PDF Microsoft Word - Chapter 1_6_SY.doc Molecular Orbital Diagrams (H2 and He2) One of the strengths of molecular orbital theory is its ability to describe the energy of both occupied and unoccupied molecular orbitals for a molecule. A molecular orbital diagram shows both the energy of the atomic orbitals (from the atoms that are... Molecular Orbital Theory: Types, Methods, Rules, Examples and... Molecular Orbital Theory. The Valence Bond Theory fails to answer certain questions like why He2 molecule does not exist and why O2 is paramagnetic. According to the Molecular Orbital Theory, individual atoms combine to form molecular orbitals. Thus the electrons of an atom are present in...
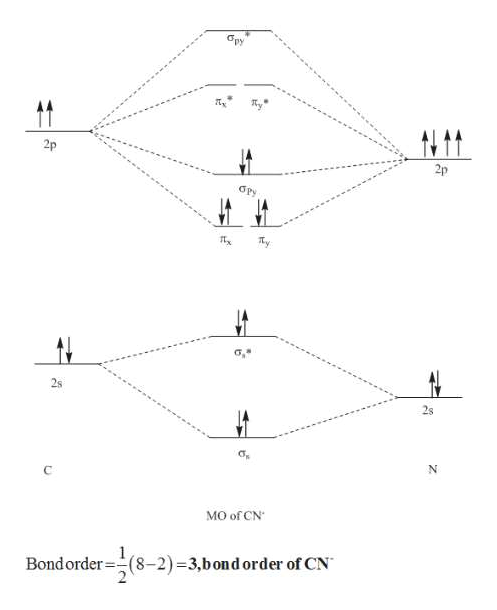
Molecular orbital diagram for h2 2-. Answered: Construct the molecular orbital diagram… | bartleby Science Chemistry Q&A Library Construct the molecular orbital diagram for H2−. Identify the bond order. Solved Construct the molecular orbital diagram for... | Chegg.com Transcribed image text : Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: 0 0.5 1 1.5 2 Click within the blue boxes to add electrons. Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... MO diagram of homonuclear diatomic molecules. The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. Molecular Orbital Theory - BH3. The BH3 molecule exists in the gas phase, but dimerizes to B2H6 (which we will look at a bit...
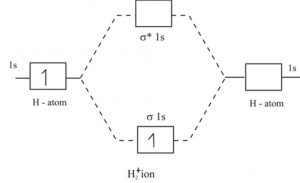
Chapter 10 : Chemical Bonding II: Molecular Shapes, valence... | Quizlet We can represent the molecular orbital energy diagram with a molecular orbital electron configuration (which is analogous to the electron configurations we wrote for elements in Section 8.3). H2 (s1s) 2 In this notation, the s1s represents the molecular orbital, and the superscript 2 represents... Energy level diagram for Molecular orbitals - Chemical Bonding and... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. Bond order value of 1 means that two hydrogen atoms are connected by a single bond. Greater value of bond order for H2 molecule than H2+ ion shows... Chapter 6 - Molecular Structure In molecular orbital theory, atomic orbitals on different atoms are mixed to produce bonds that can be localized between two atoms but are frequently As an example of the use of a diagram such as the one shown in Figure 6.21, we examine the differences predicted for the H2 and He2 molecules. PDF Figure 9.32: The molecular orbital energy-level diagram for • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the H2 molecule. (b) The shapes of the molecular orbitals are obtained by squaring the wave functions for MO1 and...
Molecular Orbital Theory | Boundless Chemistry In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this often, but not always, yields the same result. This MO diagram depicts the molecule H2, with the contributing AOs on the outside sandwiching the MO. How to Make the Molecular Orbital Diagram for H2(2-): Does the... This video discusses how to draw the molecular orbital (MO) diagram for the H2(2-) molecule. The bond order of H2(2-) is calculated and the meaning of this... MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Is H2 a viable molecule for the molecular orbital theory? - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
Figure 14: The molecular orbital energy-level diagram for diatomic... Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding (1σ) and antibonding (2σ) molecular orbitals they form.
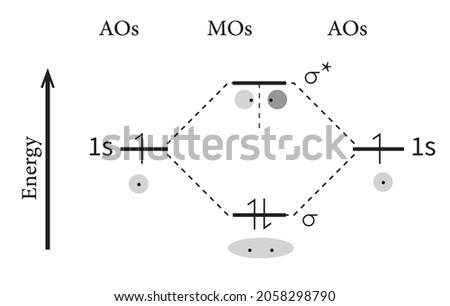
Molecular Orbital Theory The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. Placing an electron in this orbital therefore stabilizes the H2 molecule. This diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms.
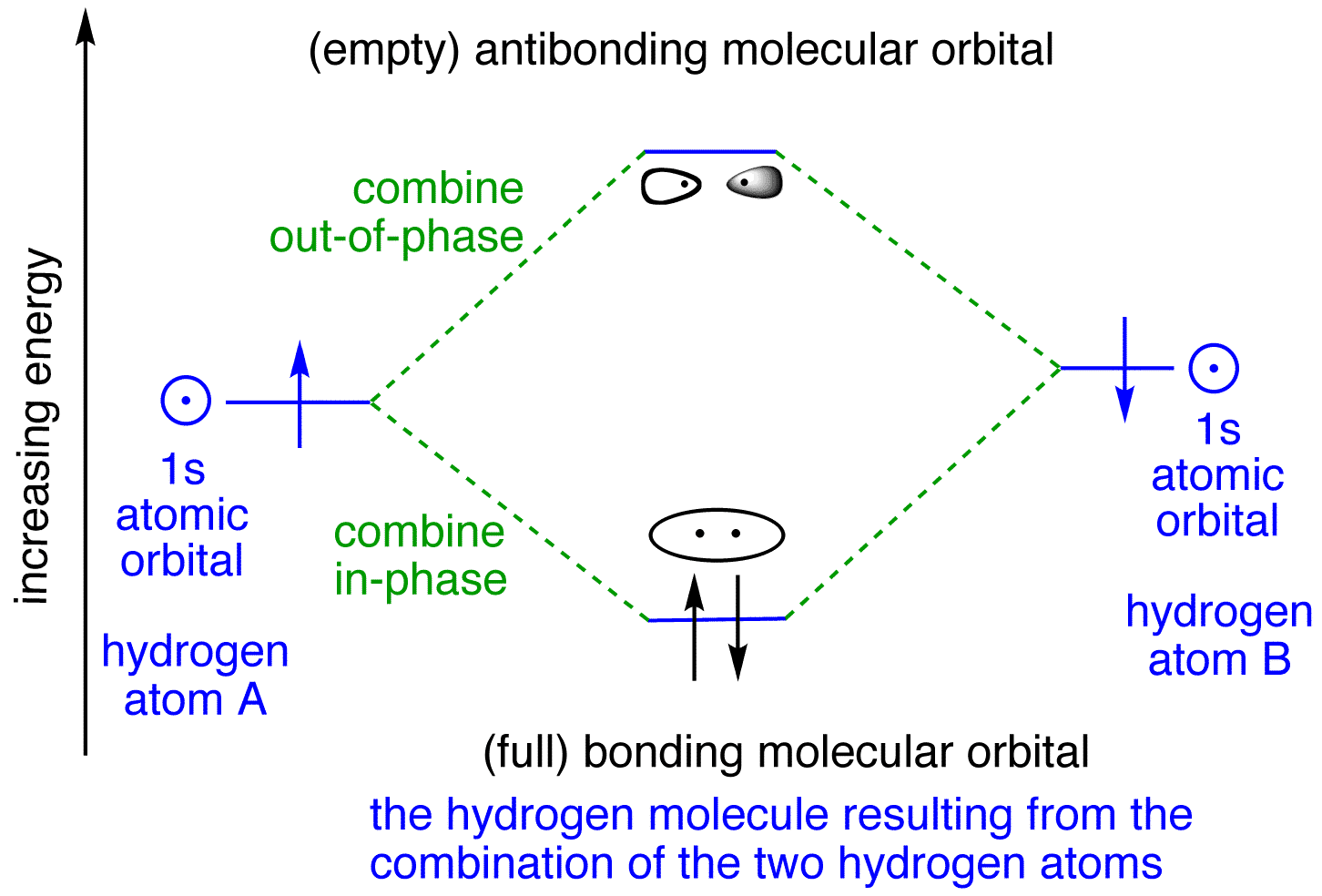
Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) About molecular orbitals. The simplest molecule: H2+. Bonding and antibonding orbitals. Simple molecular orbital diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+.
8.4 Molecular Orbital Theory - Chemistry Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Construct SALCs and the molecular orbital diagram for H\(_2\) O. In the previous examples shown for the molecular orbital diagrams of the bifluoride anion and carbon dioxide, we discussed differences in the understanding of those molecules from molecular orbital theory compared to Lewis structures. Water contains two lone pairs in its Lewis structure.
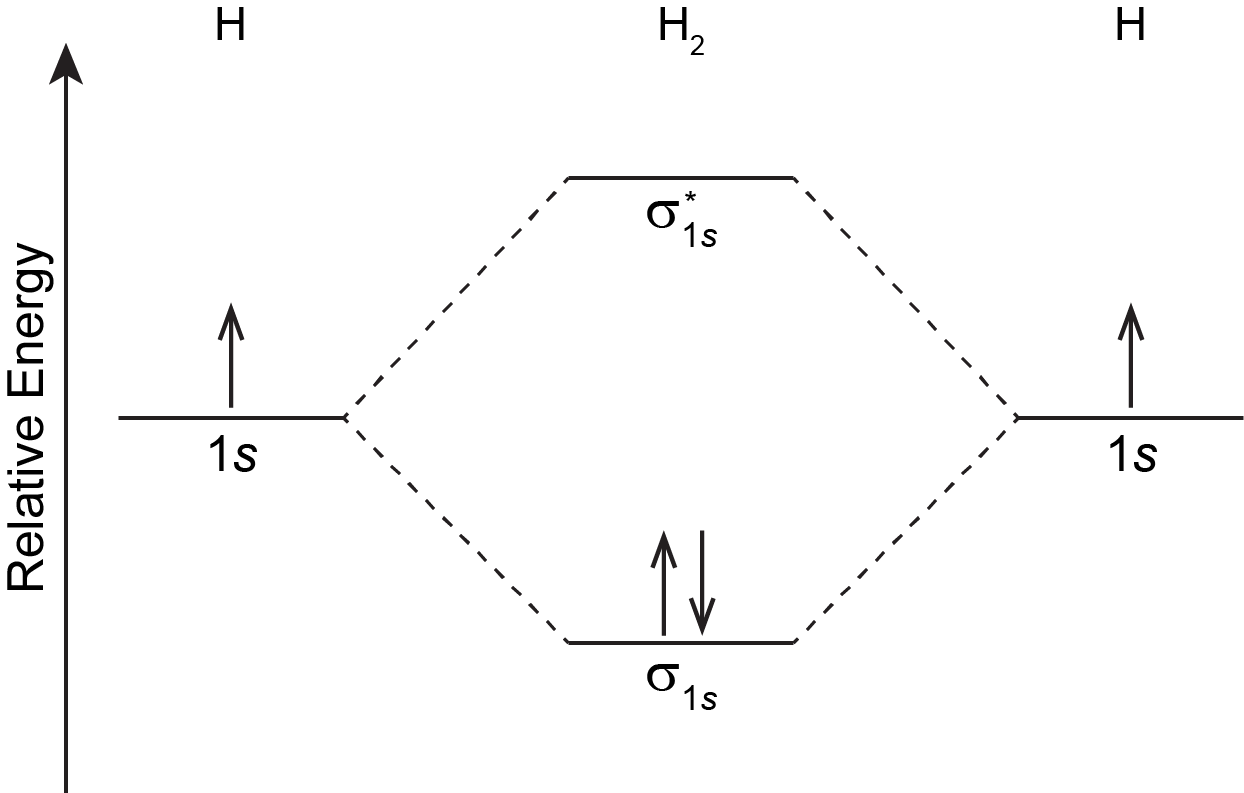
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. The energy associated with two electrons in the H2 molecule is lower than that associated with the same two electrons in separate 1s atomic orbitals.
Molecular Orbitals: Molecular Orbital Theory | SparkNotes Molecular orbital theory posits the notion that electrons in molecules likewise exist in different orbitals that give the probability of finding the electron at Notice that the orbitals of the separated atoms are written on either side of the diagram as horizontal lines at heights denoting their relative energies.
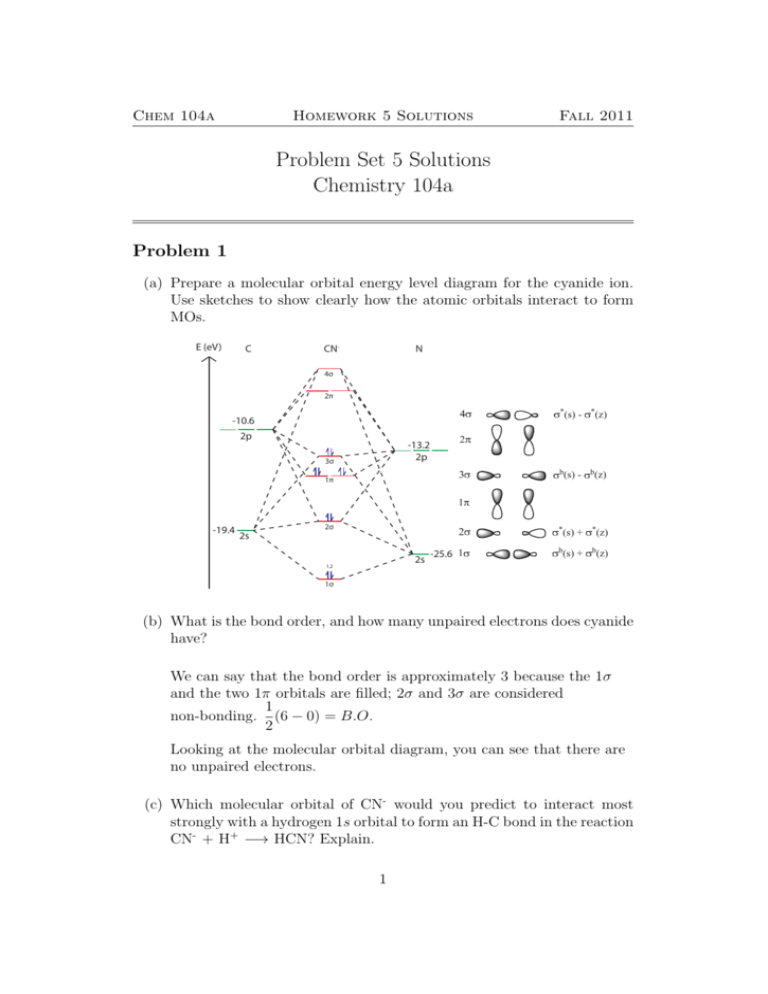
PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently Determine the primary MOs that determine the bond order. Compare the general features of your MO diagram to the MO diagram for [F-H-F]...
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... ...molecular orbitals (MOs) (σ, π and g, u) - Homonuclear diatomic MO diagrams - mixing of different AO's - More complex molecules (CO, H2O ….) • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital...
Molecular Orbital Theory: Types, Methods, Rules, Examples and... Molecular Orbital Theory. The Valence Bond Theory fails to answer certain questions like why He2 molecule does not exist and why O2 is paramagnetic. According to the Molecular Orbital Theory, individual atoms combine to form molecular orbitals. Thus the electrons of an atom are present in...
PDF Microsoft Word - Chapter 1_6_SY.doc Molecular Orbital Diagrams (H2 and He2) One of the strengths of molecular orbital theory is its ability to describe the energy of both occupied and unoccupied molecular orbitals for a molecule. A molecular orbital diagram shows both the energy of the atomic orbitals (from the atoms that are...
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Figure 8.35 The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.



















0 Response to "38 molecular orbital diagram for h2 2-"
Post a Comment