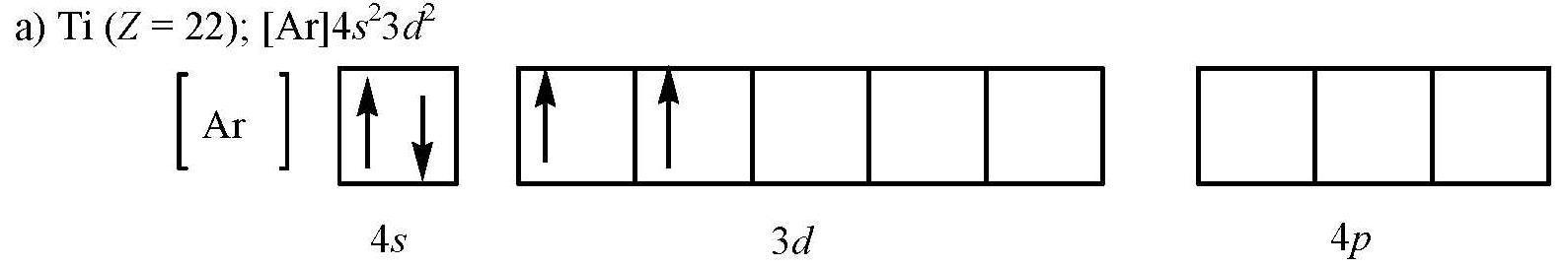
37 orbital diagram of ti
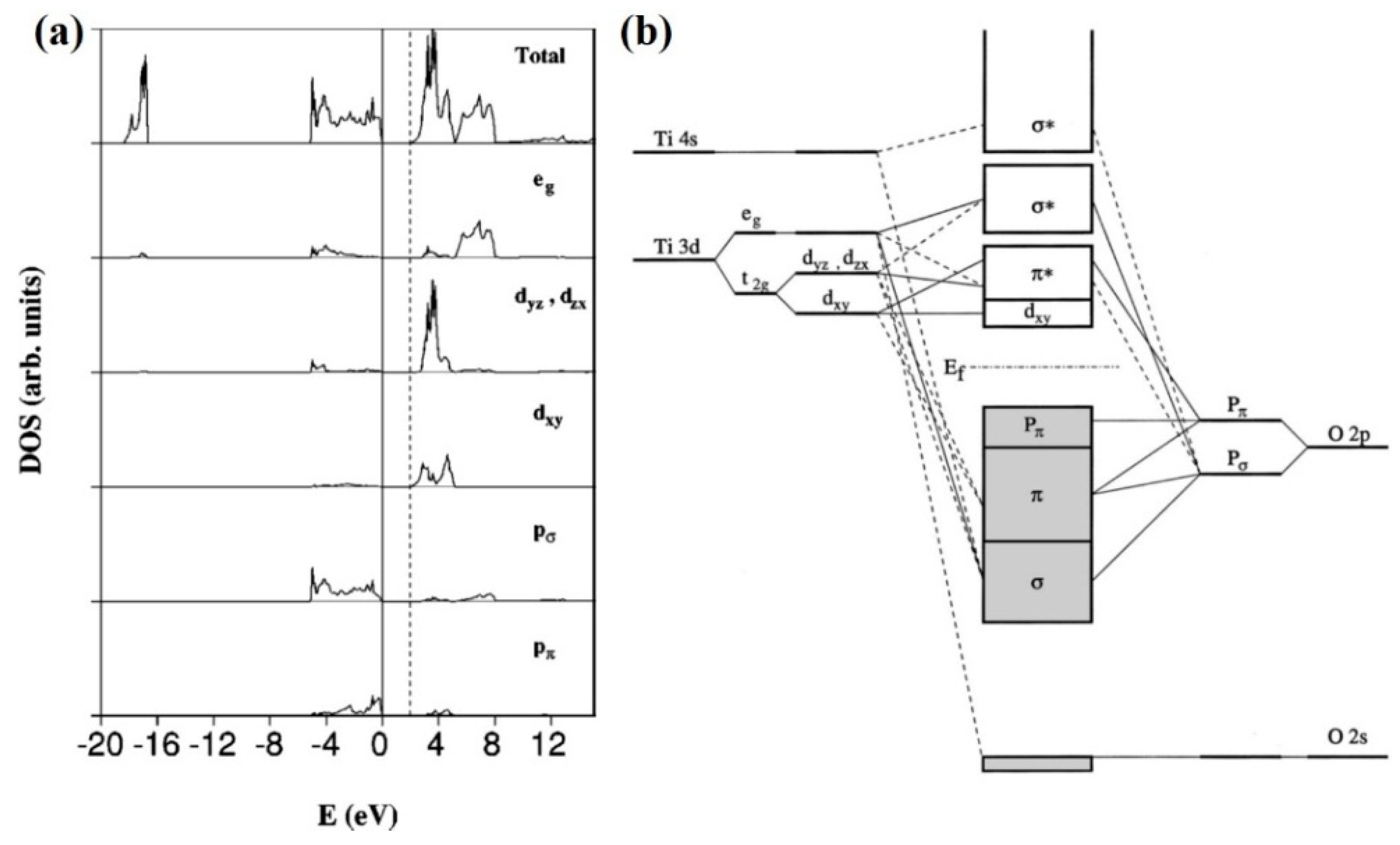
It adds its next electron to the third shell, not the outermost fourth shell. With a configuration of 2-8-10-2, titanium is out in the world and ready to bond with other elements. It makes many natural compounds with halogens and oxygen. Since titanium is out there with four extra electrons, it is quite flexible and forms many compounds. Molecular orbital energy-level diagram of anatase TiO2 (adapted from Asahi et al.181). (b) Calculated total density of states (top) and projected density of states for Ti 3d and O 2p orbitals ...
Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium ...

Orbital diagram of ti
Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+ Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+ 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... Problem Details. Construct the orbital diagram of each atom or ion. Q. Write the corresponding electron configuration for the following pictorial representation. Give the full electron configuration. Name the element, ass... Q. Create the atomic orbital diagram for nitrogen. Titanium (Ti) has an atomic mass of 22. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
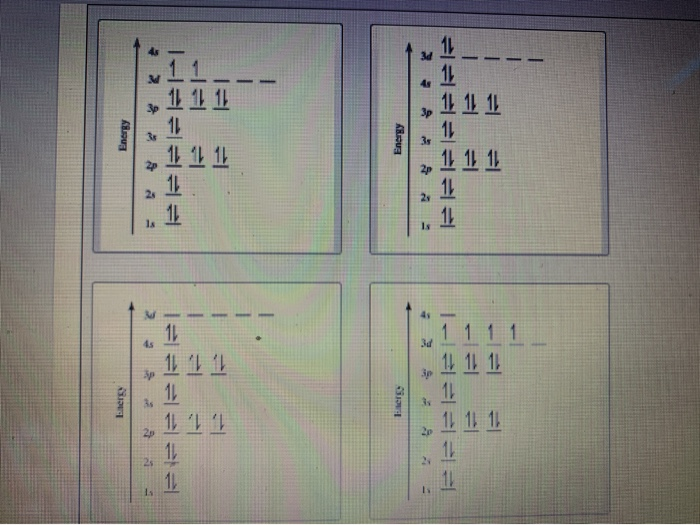
Orbital diagram of ti. Ti takes an oxidation state of 4+. In its ground state it is [Ar]3d24s2 if you subtract the 4 electrons it is simply the electron config of Argon or [Ar]. Construct the orbital diagram of each atom or ion. Ti. Ti 2+ Ti 4+ Next. Practice Problems. Choose the orbital diagram that represents the gro Consider the portion of the orbital filling diagra Choose the orbital diagram that represents the gro Identify the element which has the following parti. Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+. What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.
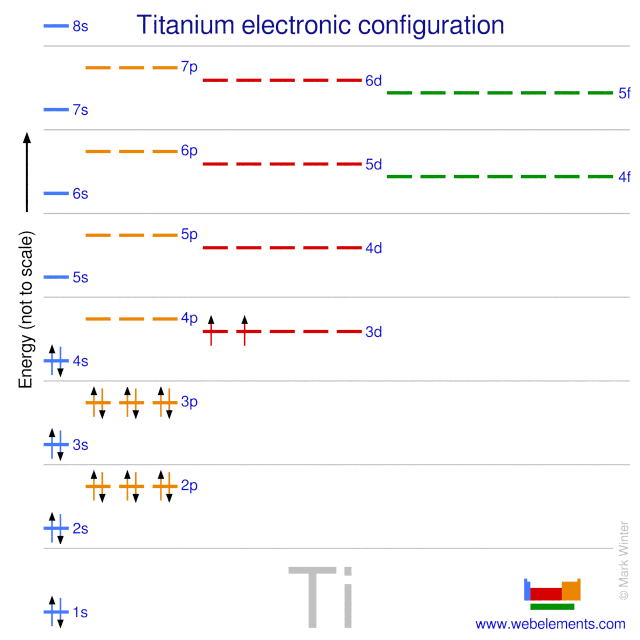
What is the orbital diagram of TI? Electrons & Oxidation. Oxidation States. +4,3,2. Electrons Per Shell. 2 8 10 2. Electron Configuration. [Ar] 3d2 4s2. 1s2 2s2 2p6 3s2 3p6 3d2 4s2. Orbital Interaction Diagram 1. Plot atomic valence orbital en ergies (or fragment orbitals for More complex molecules). 2. Determine which orbitals can interact (those with S 0). 3. Determine magnitude of each interaction: scales directly with magnitude of overlap scales inversely with orbital energy difference 4. Plot MO energies and draw orbitals Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. What is the orbital diagram for Ti 2+?
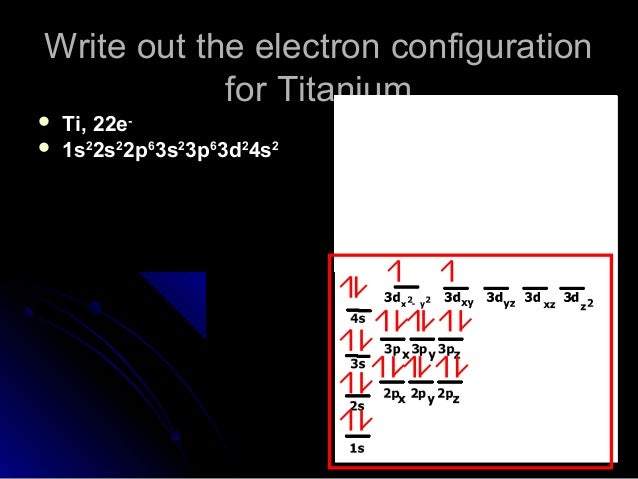
The molecular orbital diagram of TiO adapted from Ref. 6. ... the infrared data obtained in this work are combined with data for 46−50 Ti 16 O and 48 Ti 18 O at optical and microwave frequencies ... Orbital diagram for ti 4 The Aufbau Principle (also called the building-up principle or the Aufbau rule) allows us to reliably predict the ground state electron configuration of atoms and ions. The Aufbau principle states that, in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy level before ... Ti: 1s22s22p63s23p64s25d2 . It has a total of 22 electrons occupying these orbitals of these quantum numbers. Using up and down arrows, write the orbital diagram for the Ti22 atom and identify which are unpaired electrons and determine how many unpaired electrons there are.
Orbital Diagram For Titanium. First you'd follow the filling of orbitals in accordance with the Aufbau principle. This video shows how to draw the orbital diagram of Titanium (Ti). Orbital Diagram For Ti2 — UNTPIKAPPS (Nicholas Hudson) Fill in the electron configurations for the elements given in the table.
See the answer See the answer done loading. Construct the orbital diagram of each atom or ion. Ti. Ti2+. Ti4+. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Exam 4 Review: Ch.8-9. Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye. The energy of one photon of this light is ________ J. Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.19 × 10^14 Hz. Nice work!
What is the orbital diagram for {eq}\rm Ti^{2+} {/eq}? Electron Configuration of Transition Metals. Electrons of an atom are arranged in shells. Within a shell are subshells, with s, p, d, and f ...
"Ti"^(2+): ["Ar"]3d^2 A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom. In this case, titanium, "Ti", is located in period 4, group 4 of the periodic table and has an atomic number of 22. This means that a neutral titanium atom will contain 22 protons in its nucleus and 22 electrons surrounding its ...
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of ...
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is ...
Construct the orbital diagram of each atom or ion. 91 381 ratings problem details. This video shows how to draw the orbital diagram of titanium ti. To write the configuration for the titanium ions first we need to write the electron configuration for just titanium ti. Get the detailed answer. Ti ti2 ti4 switch to.
Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals.
Titanium (Ti) has an atomic mass of 22. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Problem Details. Construct the orbital diagram of each atom or ion. Q. Write the corresponding electron configuration for the following pictorial representation. Give the full electron configuration. Name the element, ass... Q. Create the atomic orbital diagram for nitrogen.
Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+ Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+ 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ...

Drag Each Image To The Correct Location On The Model Each Image Can Be Used More Than Once Apply Brainly Com

Draw The Partial Valence Level Orbital Diagram And Write The Symbol Group Number And Period Number Of The Element Ar 4s 2 3d 10 4p 3 Image Src Orbital9195593143458043682 Jpg Alt Orbital C Study Com

1 Tuliskan Bentuk Konfigurasi Elektron Dan Diagram Elektron Dari Atom Dan Ion Berikut A 27c0c 20 Brainly Co Id



















0 Response to "37 orbital diagram of ti"
Post a Comment