38 bohr diagram for sodium
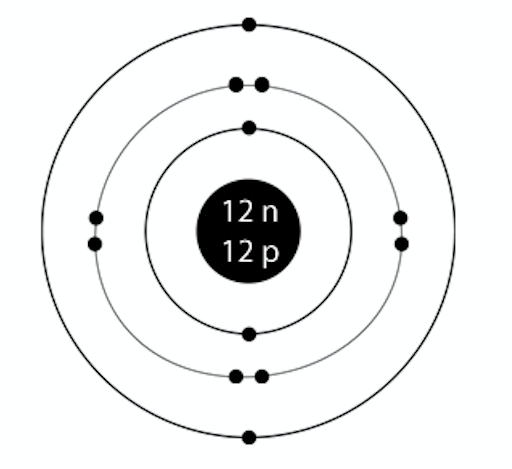
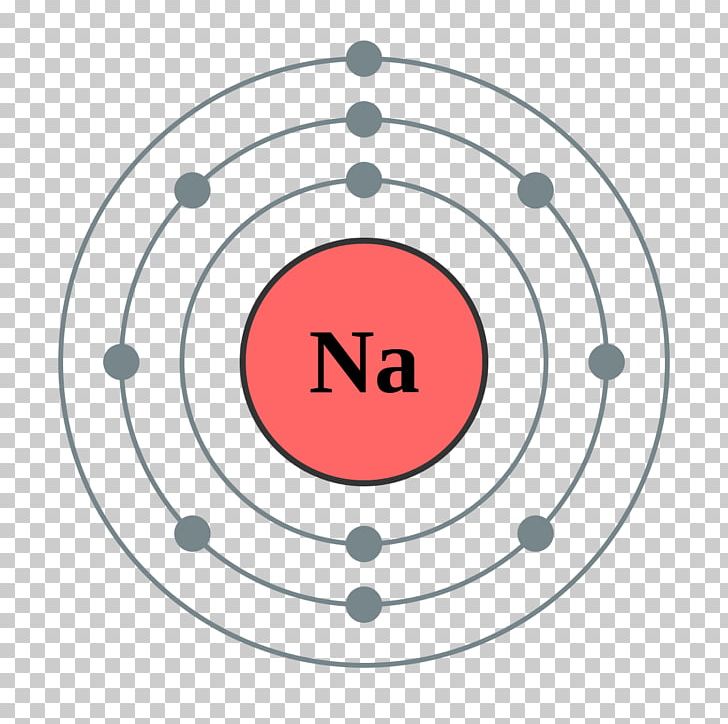
Similarly one may ask, what is the atomic structure for sodium? Sodium (Na).Diagram showing the nuclear composition and electron configuration of an atom of sodium-23 (atomic number: 11), the most common isotope of the element sodium.The nucleus consists of 11 protons (red) and 12 neutrons (blue).. Also Know, what is the atomic number of Na? STEP 2. Do the electronic configuration for the atom of the element. We always do electronic configuration for the number of electrons present in the atom of the element. The number of elecrons each shell of an element can old is given by 2n2 rule given by Bohr where n refers to the number of shell. STRUCTURE FOR SODIUM. Atomic Number:- 11.
A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. To draw the Bohr diagram for "NaCl", we should first draw the individual diagrams for both "Na" and "Cl". The atomic number of "Na" is 11, so it has 11 electrons.

Bohr diagram for sodium
The Element Sodium-- Sodium Atom. Sodium is a chemical element in the periodic table that has the symbol Na (Natrium in Latin) and atom number 11. Sodium is a soft, waxy, silvery reactive metal belonging to the alkali metals that is abundant in natural compounds (especially halite). Keeping this in view, what is a Bohr diagram? sodium atom sodium ion argon atom chlorine atom chlorine ion potassium atom potassium ion Atomic number Number of 10 protons 10 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons ... NaCl, sodium chloride, is an IONIC compound, which means electrons are TRANSFERRED from one atom to another.You'll have to draw the Bohr-Rutherford Diagram o...
Bohr diagram for sodium. Bohr Diagram Of Sodium. Photo by . Bert Hardy. on . Getty Images · Bohr Diagram Of Sodium. Christy Sego. 115 followers ... Bohr model of hydrogen atom, postulates, energy levels, calculation of radius, velocity, emission or absorption energy of electron by Bohr's theory. Bohr Diagram Of Sodium. Posted on April 19, 2019. April 18, 2019. Sponsored links. Utility Trailer Wiring Diagram. 2009 Kia Rio Fuse Box Diagram. Er Diagram To Relational Schema. 28 Led Clock Timer Circuit Diagram. Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons Below is an illustration of the Bohr model of a sodium atom. If you look at the diagrams of the sodium and chlorine atoms you can see that sodium normally has. Bohr-Rutherford diagram of sodium chloride? Bohr-Rutherford diagram of The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2.
Bohr Diagram Of Sodium. Bohr Model Of Sodium Pdfshare. Bohr Model Sodium Atom Chemistry Rutherford Model Png. Ppt Atomic Structure Powerpoint Presentation Id5055482. Using The Aph Periodic Table To Determine Valence. Bohr Rutherford Diagram For Sodium. Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8 ... Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons ... Bohr Diagram for Sodium and Chlorine Now look carefully at the following Bohr models of sodium and chlorine. 1s 22s 22p x22p y22p z23s 1. Bohr Model of a Sodium Ion Na 1 Note. See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Atom Model The Number Of Protons Is 47 And The Number Of Neutrons Is ...
Example: Determine the formula of a compound formed by the reaction of sodium and fluoride. Solution: First examine the electron arrangement of the sodium and fluorine atoms. Symbol: Atomic No. Bohr diagram: Group No. Lewis Dots: Na: 11: 2 - 8 - 1: 1: 1: F: 9: 2 - 7: 7: 7: Write the Lewis symbol for each atom. See Graphic on the left. Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The Bohr Model of Sodium(Na) has a nucleus that contains 12 neutrons and 11 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sodium contains only 1 electron that also called valence electron. •When elements form compounds, changes occur in the arrangement of electrons in the outer orbit. • Electrons are gained or lost so that element can have a stable electron arrangement of the closest noble gas. • Atoms prefer a completely filled outer shell with electrons • In order for a compound to be stable, it must have a completely filled ...
The Structure of an Atom: A Bohr model is a way to show the structure of an atom in regards to its subatomic particles. ... A Bohr model for sodium shows that it has eleven protons and neutrons ...
Diagrams - Public Service Announcement: Sodium Fluoride. Lewis Dot Diagram- The sodium transfers one electron to the fluorine so they can both become stable. Bohr-Rutherford Diagram: Sodium is the cation as it has a positive charge after losing one electron, while fluoride is the anion as it has a negative charge after gaining one electron.
which for sodium is 11. The number of neutrons is equal to the mass number (rounded to the nearest whole number- the number of protons. So for sodium it is 23 minus 11 which is equal 12. Put these numbers into your diagram. Bohr Diagram for Sodium x x x x x x x x x x x 11 P 12 N
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom.
The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. Since Sodium's Atomic Number is 11, that is also the number of electrons. The first energy level can hold 2 electrons, the next 8, and the third So the diagram has two ele ctrons on the first level, eight on ...
Bohr won a Nobel Prize in Physics for his contributions to our understanding of the structure of atoms and how that is related to line spectra emissions. Key Concepts and Summary. Bohr incorporated Planck's and Einstein's quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra.
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.
Sodium has 2 electrons in its first shell, 8 in its second and 1 in its third.Check me out: http://www.chemistnate.com
This analysis shows that sodium has only one electron in its outer level. The nearest rare gas is neon with 8 electron in the outer energy level. Therefore, this electron is lost so that there are now eight electrons in the outer energy level, and the Bohr diagrams and Lewis symbols for sodium ion and neon are identical.
In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902 ...
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
NaCl, sodium chloride, is an IONIC compound, which means electrons are TRANSFERRED from one atom to another.You'll have to draw the Bohr-Rutherford Diagram o...
sodium atom sodium ion argon atom chlorine atom chlorine ion potassium atom potassium ion Atomic number Number of 10 protons 10 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons ...
The Element Sodium-- Sodium Atom. Sodium is a chemical element in the periodic table that has the symbol Na (Natrium in Latin) and atom number 11. Sodium is a soft, waxy, silvery reactive metal belonging to the alkali metals that is abundant in natural compounds (especially halite). Keeping this in view, what is a Bohr diagram?
GCSE CHEMISTRY - What is a Sodium Ion? - How do you Draw a Sodium Ion?- What is the Electronic Structure of a Sodium Ion? - GCSE SCIENCE.

Student Notebook Containing Notes, Diagrams and Swatches (c. 1898–1900) // Alfred Fehr (Switzerland, 1879-1955)

Plan of Chicago, Chicago, Illinois, Diagram Showing City Growth (1909) // Daniel Hudson Burnham (American, 1846-1912) Edward Herbert Bennett (American, born England, 1874-1954)

Plate 75 from The Plan of Chicago, 1909: Chicago. Diagram of the City, Showing Complete System of Inner Circuits (1909) // Daniel Hudson Burnham, American, 1846-1912 Edward Herbert Bennett, American, born England, 1874-1954

Marina City Theater, Chicago, Illinois, Roof and Partial Concrete Frame Development Drawing (1961-1962) // Bertrand Goldberg American, 1913-1997





















0 Response to "38 bohr diagram for sodium"
Post a Comment