38 lewis diagram for n2
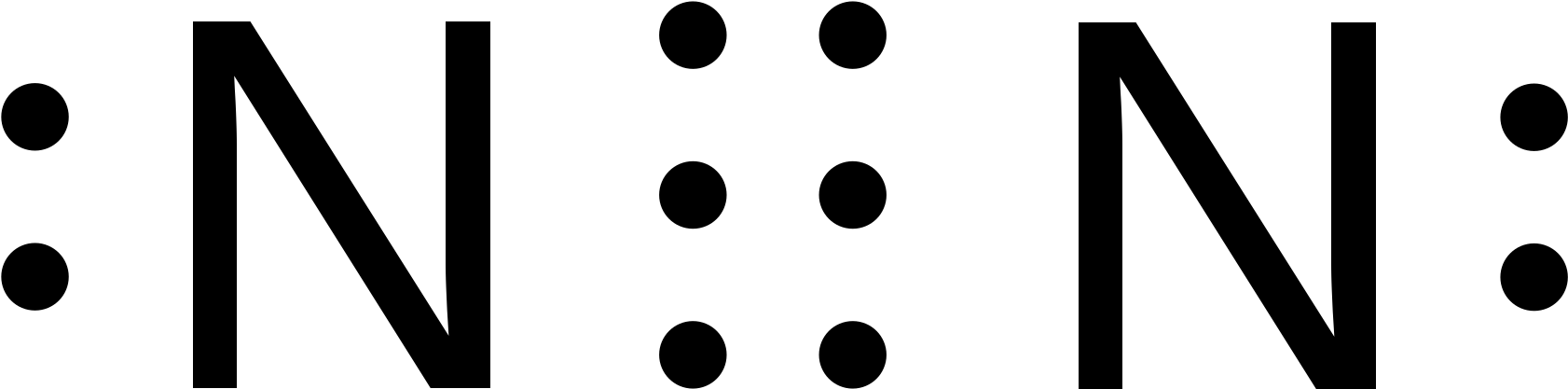
N2 Lewis Structure Setup. It’s easiest to think in terms of dots to make the N 2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won’t bond, on top of each N. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . The Lewis dot structure for any molecule can be found by following a general set of This is derived by following 5 general steps for writing Lewis dot structures.
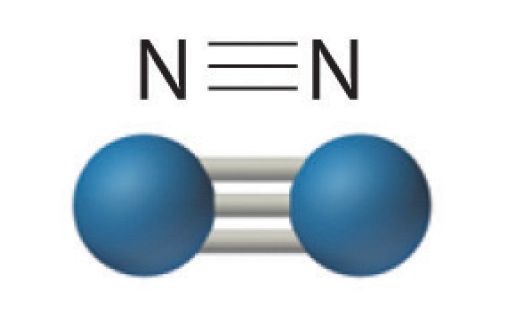
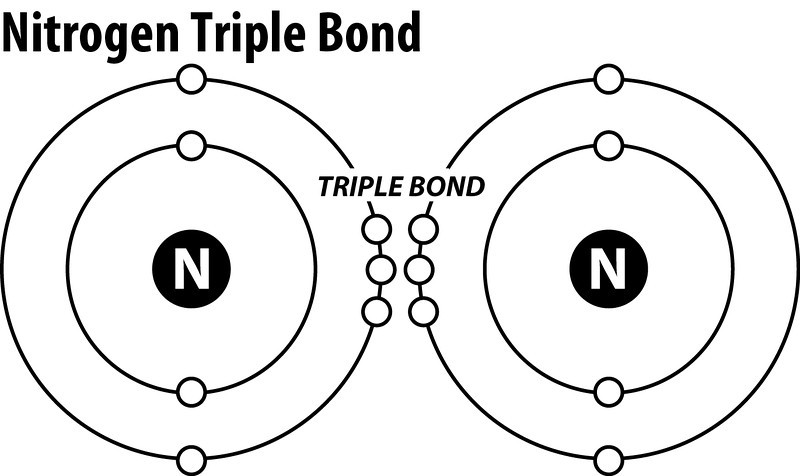
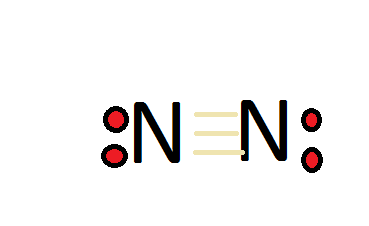
In the lewis structure of N2, there is a triple bond between two nitrogen atoms. The molecular geometry of N 2 is linear. N2 is colorless, odorless, and tasteless gas. Each nitrogen atom is surrounded by a lone pair of electrons. There are three half-filled 2p orbitals in the valence shell of the nitrogen atom.
Lewis diagram for n2
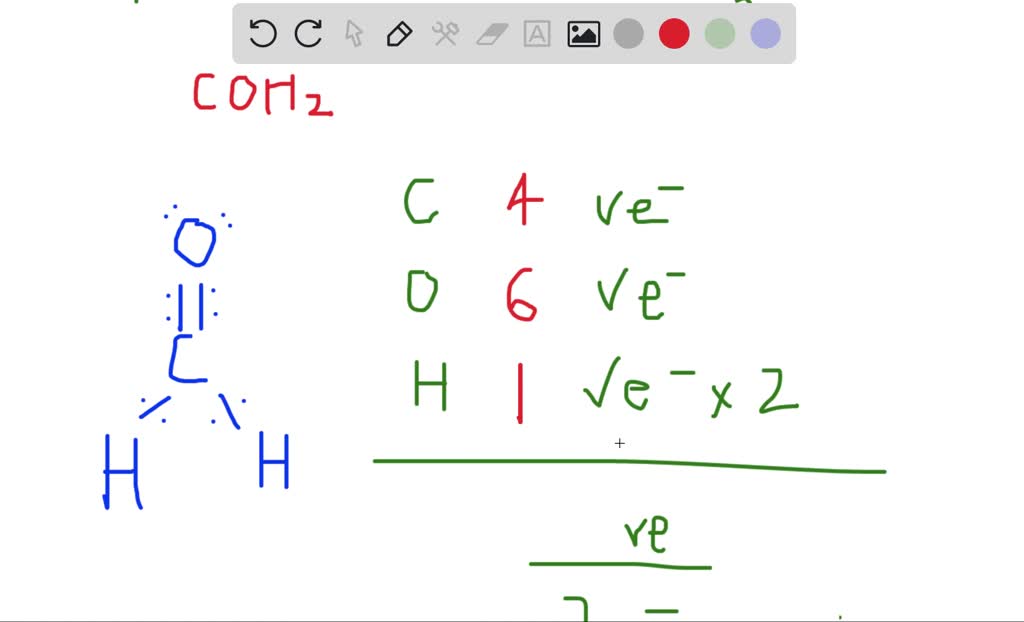
N2 Lewis Structure In the N2 molecule, there are only two atoms are present. According to the periodic table, each n2 nitrogen comes with the 5 valence electrons. The nitrogen molecule contains 2 nitrogen atoms so a total of 10 valence electrons in the n2 molecule. The first step in the Lewis diagram is Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
Lewis diagram for n2. How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com A step-by-step explanation of how to draw the N2O Lewis Dot Structure (Dinitrogen monoxide or Nitrous Oxide).For the N2O structure use the periodic table to ... In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-. Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i...
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen. I quickly take you through how to draw the Lewis Structure of N2 (DiNitrogen) . I also go over the shape and bond angles. A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Diatomic Nitrogen).For the N2 structure use the periodic table to find the total number...
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond. Lewis Structure of N2 (Nitrogen gas)||Lewis Structure for N2 (Nitrogen gas)#LewisStructureofN2#N2LewisStructure#LewisStructureforN2This video has solved the ... lewis structure n2 Nitrogen is a triple bonded molecule. Since Nitrogen belongs to the diatomic molecule in the VA family, on the periodic tables, which means that the valency of the molecule is five, therefore, it needs three more valences of electrons in order to complete its octet, and therefore, it is a triple bonded molecule. Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2
Solved Question Answer The Following Questions About N2 And N2h4 A In The Box Below Draw The Complete Lewis Electron Dot Diagram Of N2 B Bas Course Hero
Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
N2 Lewis Structure In the N2 molecule, there are only two atoms are present. According to the periodic table, each n2 nitrogen comes with the 5 valence electrons. The nitrogen molecule contains 2 nitrogen atoms so a total of 10 valence electrons in the n2 molecule. The first step in the Lewis diagram is

Write The Molecular Orbital Diagram Of N2 And Calculate Their Bond Order Chemistry Topperlearning Com Qbqjy

Solved N2 Lewis Structure Nitrogen Is Nitrogen N2 Polar Or Nonpolar Molecular Geometry And Bond Angle Of Nitrogen Solution Platform
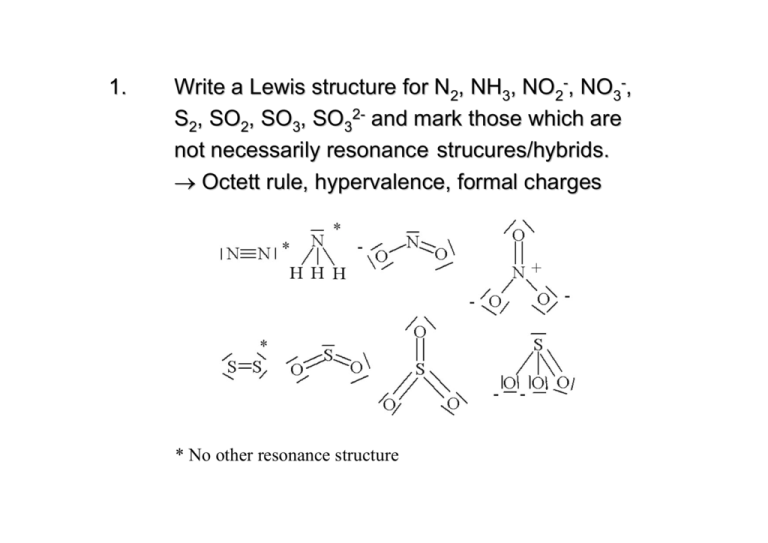
Solved Draw The Lewis Structure Of Each Of The Following Molecules O2 N2 Cn And H2o Then Determine The Bond Order And Multiplicity Of Each Of The Molecules Listed Below Based On A



















0 Response to "38 lewis diagram for n2"
Post a Comment