39 the orbital diagram for a ground state nitrogen atom is
The orbital diagram for a ground state nitrogen is a a b b c c d d the electron configuration of an atom shows a the number of isotopes possible. C has two unpaired electrons in its ground state. The following orbital filling diagram represents an excited state rather than the ground state of an atom. It depends on the atom. The orbital diagram for a ground state nitrogen atom is. C has two unpaired electrons in its ground state. A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is a. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.
The orbital diagram for a ground state nitrogen is a a b b c c d d the electron configuration. It depends on the atom. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. The ground state electron configuration of p is ne3s23p3. Nitrogen is the seventh element with a total of 7 electrons.
The orbital diagram for a ground state nitrogen atom is
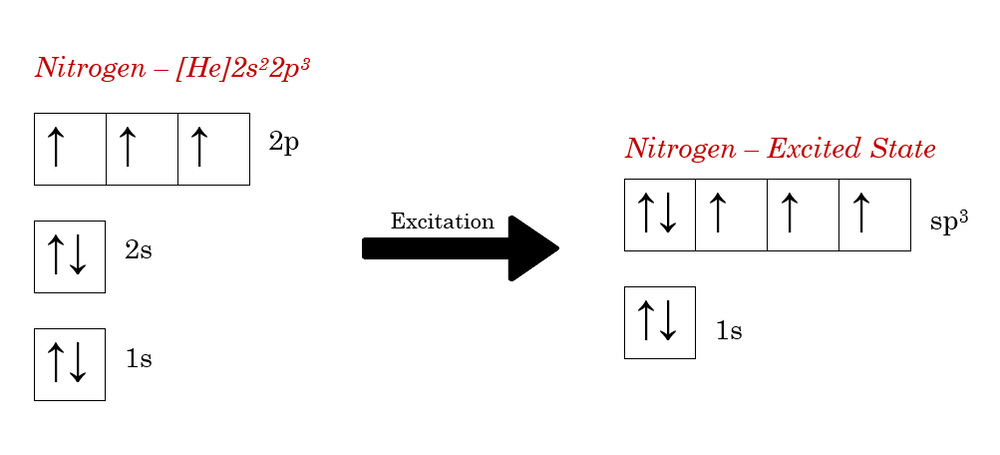
A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 z.It therefore has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), exceeded only by chlorine (3.16), oxygen (3.44 ... 15.08.2020 · We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. The neutral atom chlorine (Z=17), for instance has 17 electrons. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. 24 Oct 2021 · 4 answersHi there. In this question, we are trying to show the orbital diagram for A nitrogen atom and nitrogen is atomic # seven.
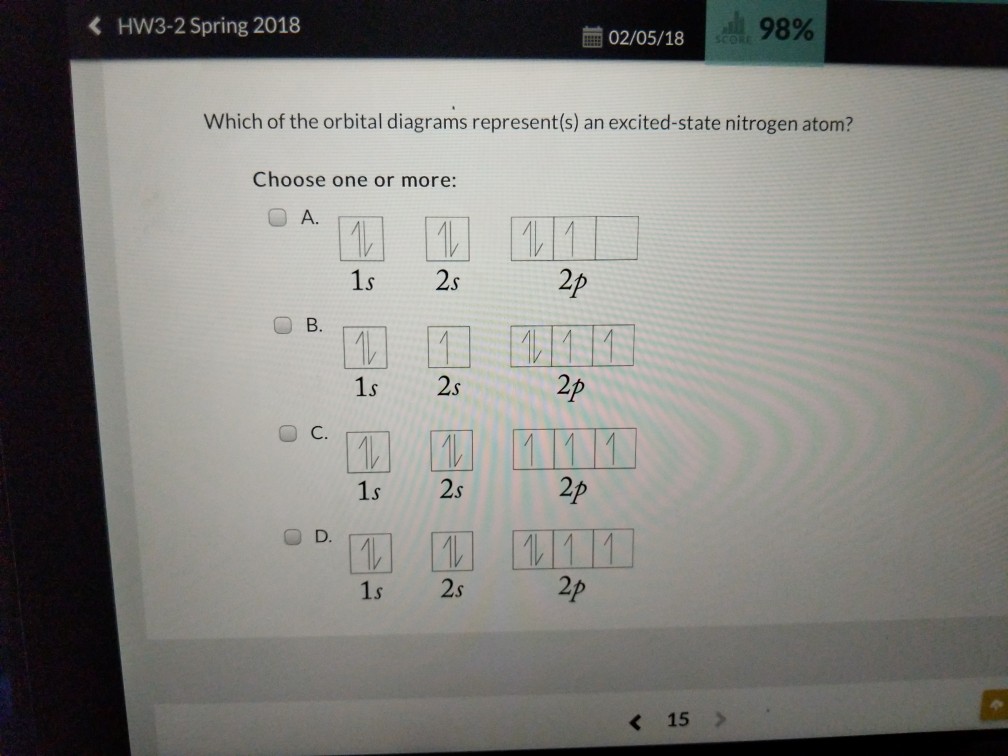
The orbital diagram for a ground state nitrogen atom is. 30.04.2018 · Developing highly active single-atom catalysts for electrochemical reactions is a key to future renewable energy technology. Here we present a … 21 Jan 2021 — 1s22s22p3 is the ground-state electron configuration for N. You can easily understand the image given below. The electron configuration of an atom shows the number of electrons in each sublevel in each energy level of the ground-state atom. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Each added electron is assigned to the lowest-energy sublevel available. The ... Transcribed image text: Question 21 The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p A. It It Î Î Î B.T ft TuI_ c. ît Î Î Î D. ît îţ îi Î Î ...
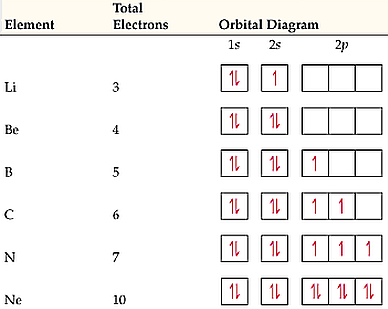
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. Chemistry questions and answers. The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소. 14.08.2020 · The electron configuration and orbital diagram for carbon are: Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule. These three electrons have unpaired spins. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in ... The orbital diagram for a ground-state nitrogen atom is. 1s (up down) 2s (up down) 2p (up, up, up) Electrons in an orbital with l = 3 are in a/an. ... For all atoms of the same element, the 2s orbital is larger than the 1s orbital. true. An FM radio station broadcasts at a frequency of 101.7 MHz. Calculate the wavelength
1 answerElectronic configuration of nitrogen in ground state is 1s2 2s2 2p3 or 1s2 2s2 2px1 2py1 2pz1. Hence, in excited state one of the 2s electron will jump to 2p ... The orbital diagram for a ground-state nitrogen atom is. Ans: A. Category: Medium Section: 7.8. 41. The orbital diagram for a ground-state oxygen atom is. Ans: D. Category: Medium Section: 7.8. 42. The orbital diagram for a ground state carbon atom is. Ans: D. Category: Medium Section: 7.8. 43. Which ground-state atom has an electron ... The orbital diagram for a ground-state nitrogen atom is. 1s 2s 2p ↑↓ ↑↓ ↑ ↑ ↑. Image: The orbital diagram for a ground-state nitrogen atom is. A nitrogen atom has 3 orbitals; the 1s orbital, the 2s orbital, and the 2p orbital. In this case, the 2s and 2p orbitals are the valence orbitals, as they have the electrons with the most energy.
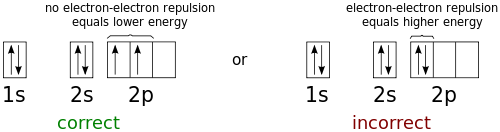
In the ground state of an atom or molecule, the unpaired electrons usually all have parallel spin. In this case the multiplicity is also equal to the number of unpaired electrons plus one. Atoms. The multiplicity is often equal to the number of possible orientations of the total spin relative to the total orbital angular momentum L, and therefore to the number of near–degenerate levels that ...
What ground state atom has an electron configuration described by the following orbital. 4 chem 101 worksheet 7 quantum theory and electronic structure ii. Answer to the orbital diagram for a ground state nitrogen atom is. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. The orbital diagram ...
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
here, it states choose the orbital diagram that represents the ground state of nitrogen. Now, if you look at your periodic table, realize that nitrogen is ...17 Sep 2020
05.11.2021 · Covalent organic polymer has been considered as the promising organic semiconductor for the photocatalytic CO 2 reduction reaction (CO 2 RR) due to its unique features such as adjustable framework, durable stability and feasible bandgap energy, et al.Herein, we reports a novel Co II-porphyrin/Ru II-pincer complex coupled polymer (CoPor-RuN 3), which exhibits efficient visible light …
24 Oct 2021 · 4 answersHi there. In this question, we are trying to show the orbital diagram for A nitrogen atom and nitrogen is atomic # seven.

Electron Configuration Excited State Molecular Orbital Diagram Ground State Energy Angle White Text Png Pngwing
15.08.2020 · We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. The neutral atom chlorine (Z=17), for instance has 17 electrons. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5.
A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 z.It therefore has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), exceeded only by chlorine (3.16), oxygen (3.44 ...
Solved Gallium Arsenide Gaas Is Used In The Red Lasers Of Bar Code Readers Write The Full Electron Configuration Of A Ground State Atom Of Galli Course Hero

The Ground State Valence Shell Electrons Configuration Of Nitrogen Atom Can Be Represnted As Youtube

Plan For Fri 7 Nov 08 Lecture Periodic Trends 7 12 Types Of Chemical Bonds 8 1 Electronegativity 8 2 Quiz Ppt Download

Intermediate Valence States In Lanthanide Compounds Tricoire 2021 Chemistry A European Journal Wiley Online Library



















0 Response to "39 the orbital diagram for a ground state nitrogen atom is"
Post a Comment