39 potential energy diagram catalyst
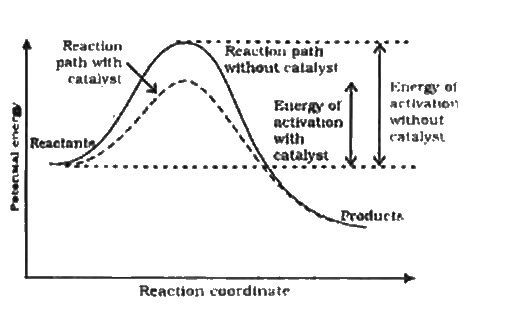
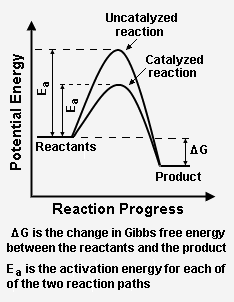
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... But, before the reactants can be converted into products, the free energy of the system must overcome the activation energy for the reaction, as shown in the figure below. The vertical axis in this diagram represents the free energy of a pair of molecules as a chlorine atom is transferred from one to the other.
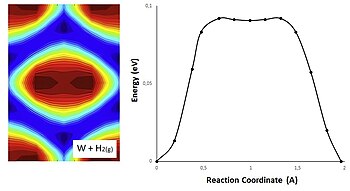
For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products. Reaction coordinate diagrams are derived from the corresponding potential energy surface (PES), which are used in computational chemistry to model chemical reactions by ...
Potential energy diagram catalyst
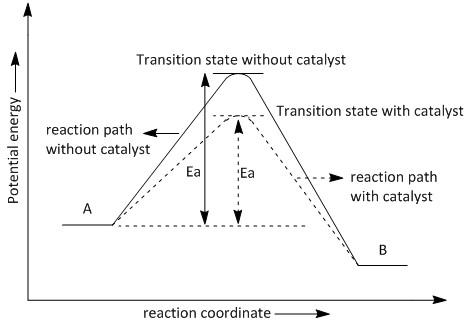
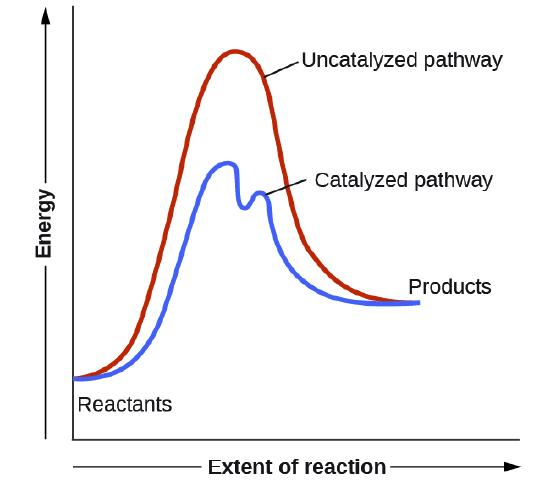
Conversion of Electric Potential Energy. STEM Activities. Next-Generation Molecular Workbench activities include one or more interactives, plus text, embedded images, video and assessments. There are currently six activities available. We're working on lots more, plus an authoring system that will allow teachers to create their own custom ... catalyst P catalyst P potential energy A B P Figure 1.2. Potential energy diagram of a heterogeneous catalytic reaction, with gaseous reactants and products and a solid catalyst. Note that the uncatalyzed reaction has to overcome a substantial energy barrier, whereas the barriers in the catalytic route are much lower. This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...
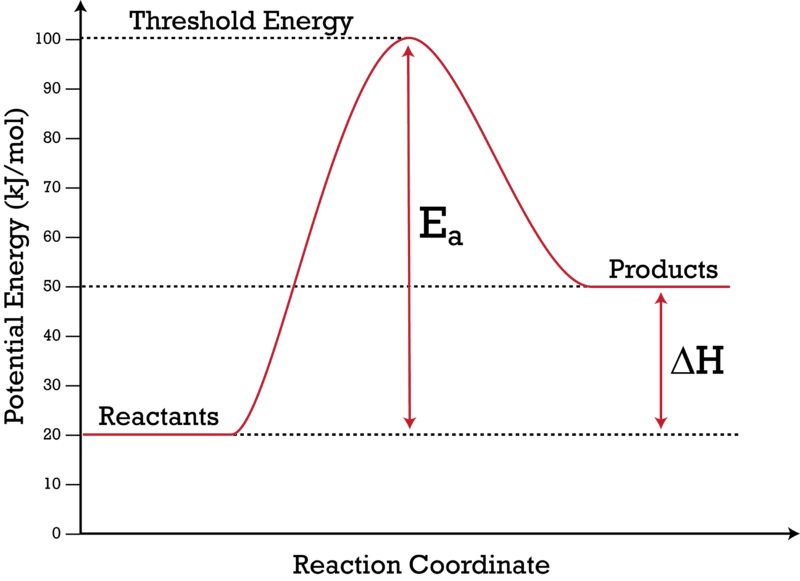
Potential energy diagram catalyst. The peak of this energy diagram represents the transition state: an intermediate stage in the reaction from which the reaction can go in either direction. Reactions with a high activation energy will proceed very slowly, because only a few molecules will obtain enough energy to reach the transition state – even if they are highly exergonic. Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Remember that the 🔼H of reaction remains the s... An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change , ΔH, is the amount of energy absorbed or released by a chemical reaction. Potential Energy Diagrams Recall, we have talked about chemical bonds having stored energy (AKA potential energy). For that reason, chemists use diagrams called Potential Energy Diagrams to illustrate the potential (or stored) energy changes that occur during specific chemical reactions. Recall: A reaction is the breaking and reforming of bonds
This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ... Jan 01, 2015 · Schematic diagram of a battery energy storage system operation. ... For example, sunlight can be captured by Ruthenium (Ru) as a catalyst, and electrons moves from the donor (marked as “D”) to the acceptor ... Overview of current and potential electrical energy storage options for various applications with their specifications. potential is raised above the equilibrium potential, oxidization is preferred. If below the equilibrium potential, reduction is preferred. 2. Pourbaix diagram One certain class of phase diagrams is displayed by the equilibrium electrical potential plotted against the pH, when some of the reactants have chemical activities that vary with pH. A catalyst, or enzyme, works with a substrate to decrease the amount of initial energy required to perform a specific chemical opperation, speeding the reaction up. Enzymes also work to increase ...
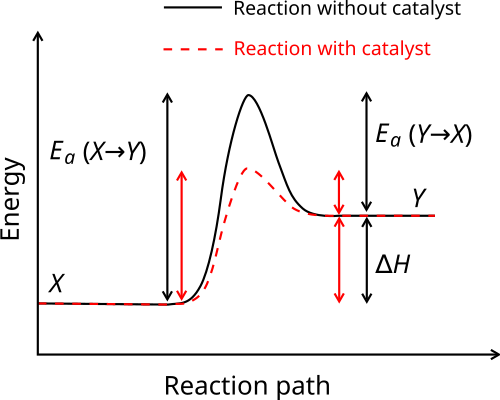
Solar Energy Technologies (SETO) 11/12/2021 05:00 PM ET : DE-FOA-0002595: Research, Development, and Demonstration Opportunities for FY 2022 Solar Manufacturing Incubator: Request for Information (RFI) Solar Energy Technologies (SETO) 11/7/2021 05:00 PM ET : DE-FOA-0002607 Why does a potential energy diagram showing the effect of a catalyst on activation energy not move left on the reaction pathway scale (compared to uncatalysed reaction) if a catalyst speeds up reac... Generic potential energy diagram showing the effect of a catalyst in a hypothetical exothermic chemical reaction X + Y to give Z. The presence of the catalyst opens a different reaction pathway (shown in red) with a lower activation energy. The final result and … Define catalyst. catalyst synonyms, catalyst pronunciation, catalyst translation, English dictionary definition of catalyst. n. 1. Chemistry A substance, usually used in small amounts relative to the reactants, that modifies and increases the rate of a reaction without being...

Draw A Graph Of Potential Energy V S Reaction Co Ordinate Showing The Effect Of A Catalyst On Activation Energy
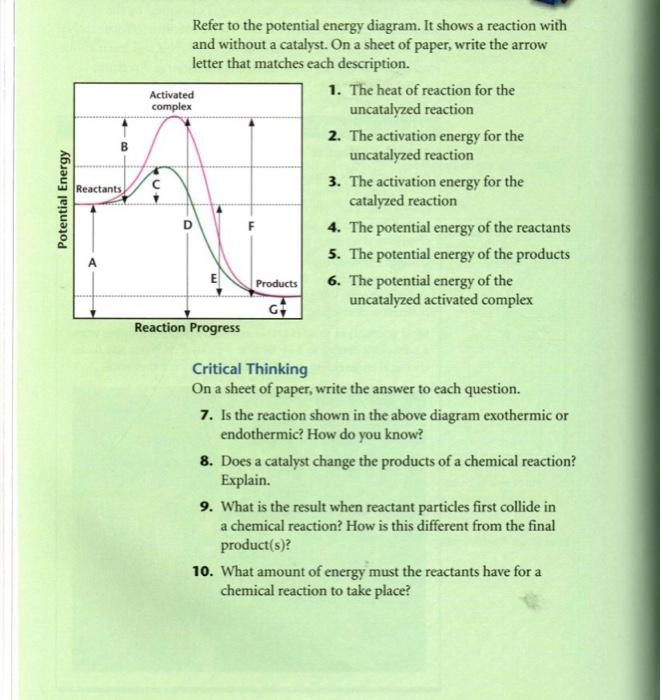
Potential Energy Diagram Worksheet ANSWERS 1. Which of the letters a–f in the diagram represents the potential ... A catalyst changes the reaction mechanism, in the process lowering the activation energy. 5. Name 4 things that will speed up or slow down a chemical reaction.
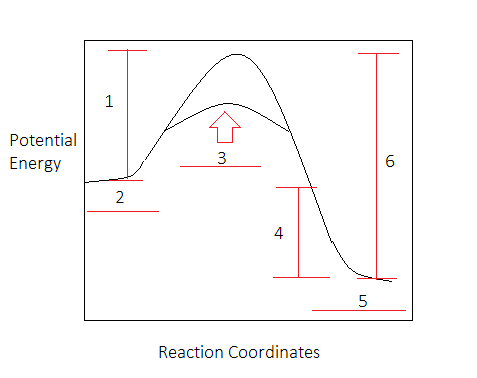
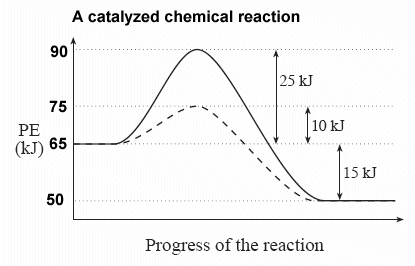
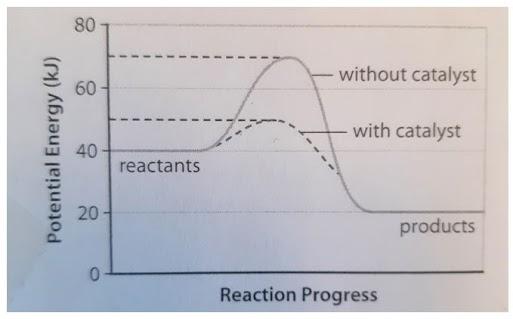
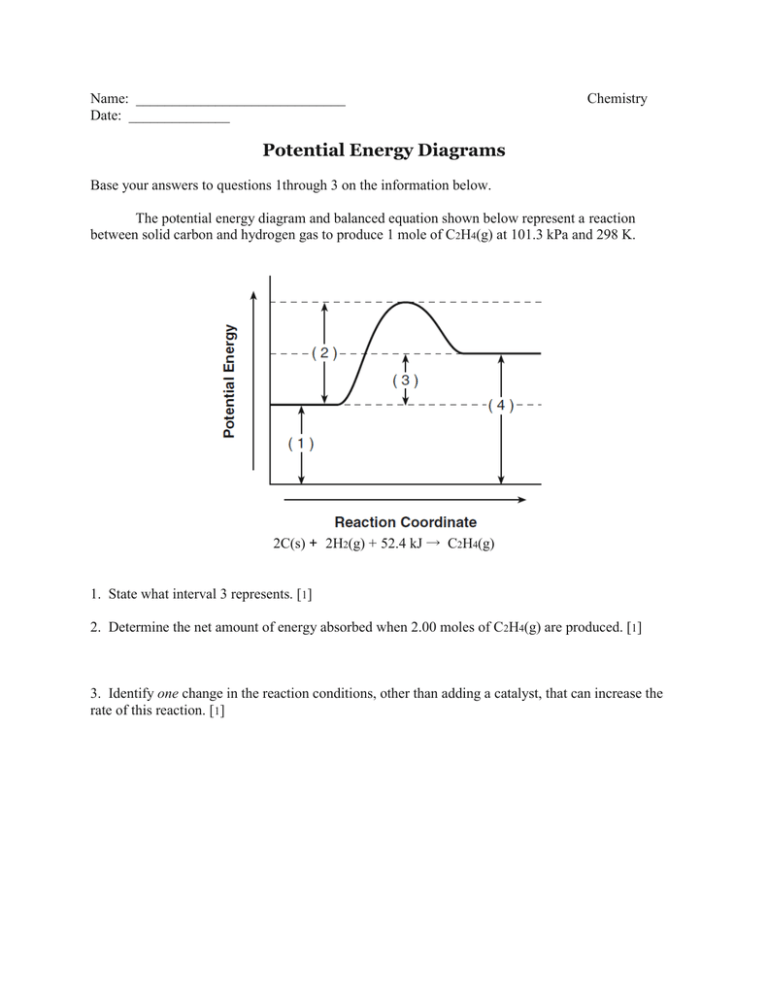
The following potential diagram is for a reaction carried out with and without a catalyst. Reaction path energy for the ca talysed Potential energy/k] mol - The activation reaction IS A c D 30 k] 80 k] 100 k] 130 k] mo I mo I mo I mo I A potential energy diagram can be used to show the activation energy (Eh) and the

Draw A Graph Of Potential Energy V S Reaction Coordinate Showing The Effect Of A Catalyst On Activation Energy
This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...
catalyst P catalyst P potential energy A B P Figure 1.2. Potential energy diagram of a heterogeneous catalytic reaction, with gaseous reactants and products and a solid catalyst. Note that the uncatalyzed reaction has to overcome a substantial energy barrier, whereas the barriers in the catalytic route are much lower.
Conversion of Electric Potential Energy. STEM Activities. Next-Generation Molecular Workbench activities include one or more interactives, plus text, embedded images, video and assessments. There are currently six activities available. We're working on lots more, plus an authoring system that will allow teachers to create their own custom ...

Explain With The Help Of A Potential Energy Diagram That The Catalyst Increases The Rate Of The Reaction Chemistry Shaalaa Com
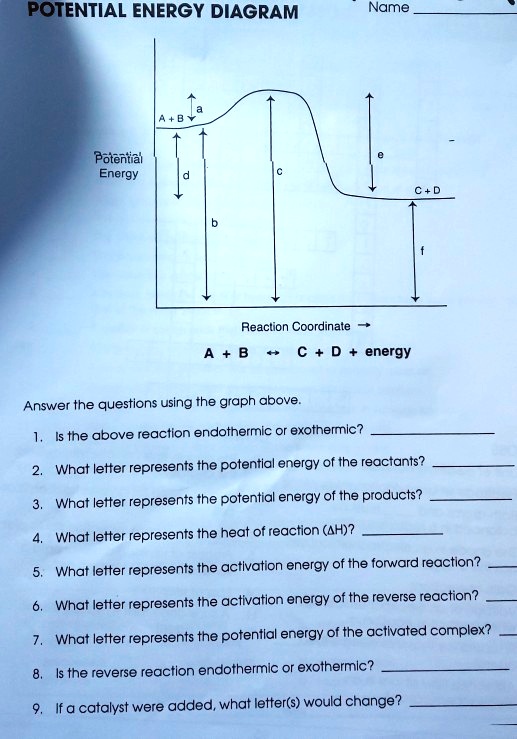
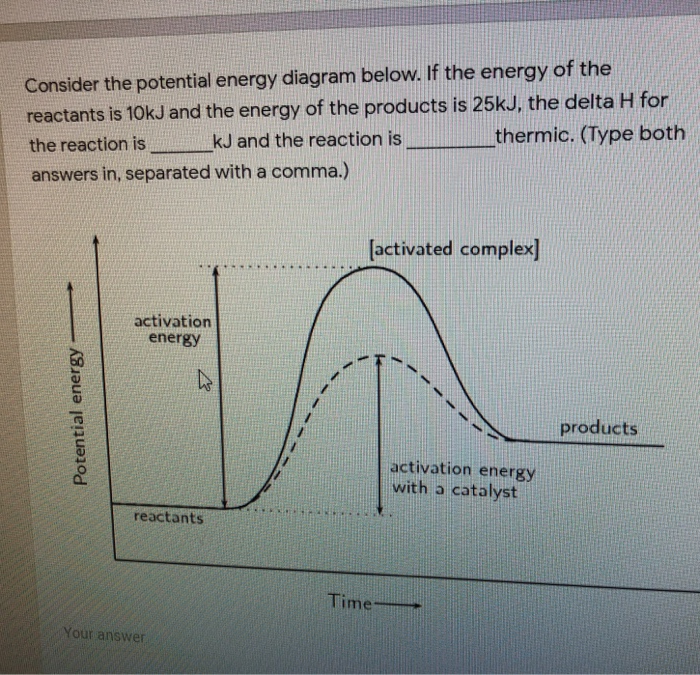
Solved Potential Energy Diagram Name Potential Energy Reaclion Coordinate C D Energy Answer The Questions Using The Graph Above Is The Above Reaction Endothermic Or Exothermlc What Letter Represents The Potential Energy

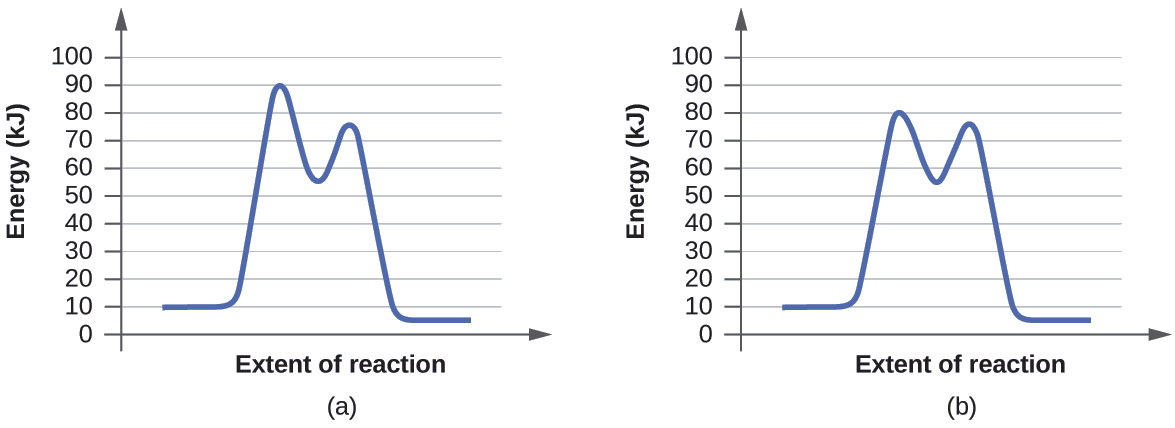
Given The Potential Energy Diagram For A Reaction Which Intervals Are Affected By The Addition Of Brainly Com
Draw A Graph Of Potential Energy V S Reaction Coordinate Showing The Effect Of A Catalyst On Activation Energy Sarthaks Econnect Largest Online Education Community

Unit 8 Kinetics And Equilibrium Catalysts Do Now Label The Parts Of The Potential Energy Diagram Ppt Download











0 Response to "39 potential energy diagram catalyst"
Post a Comment