42 how to do a bohr diagram
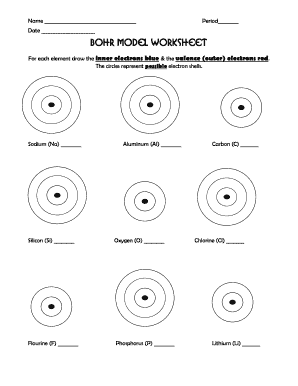
Bohr model and lewis dot diagram worksheet answers periodic table worksheets doc new blank bohr model worksheet fill in for first 20 elements and lewis dot diagram worksheet answers collection of structure with diagrams bohr and lewis models as well some naming review we marked went along the key is below if you didn t finish it homework. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Bohr diagrams are used to introduce ...
How to draw the Bohr-Rutherford Diagram for Phosphorous. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...

How to do a bohr diagram
Drawing Bohr Models 3. Draw the first shell 4. Add the electrons, the first shell can only hold 2 P:9 N:10. Drawing Bohr Models 5. Draw the next shell if you have more electrons to add P:9 N:10. Drawing Bohr Models 6. Add your electrons, the 2nd shell can hold up to 8 Be sure to add them one at a time Bohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an atom, they can be useful to help with visualizing electrons orbiting a nucleus. Drawing Bohr-Rutherford diagrams is super easy using the following steps: A step by step program for creating Bohr Diagrams and Electron dot structures. Usefule for Index Cards and Flash cards for chemistry and physical science stude… Slideshare uses cookies to improve functionality and performance, and to provide you with relevant advertising.
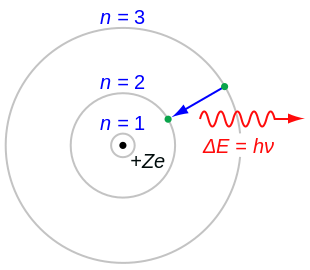
How to do a bohr diagram. Niels Bohr, a Danish scientist, explained this line spectrum while developing a model for the atom: The Bohr model shows that the electrons in atoms are in orbits of differing energy around the nucleus (think of planets orbiting around the sun). Bohr used the term energy levels (or shells) to describe these orbits of differing energy. To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number. After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells. From the periodic table, find the element, and identify its atomic number and atomic ... This photo about: How to Draw A Bohr Diagram, entitled as How To Do Bohr Diagrams How To Draw A Bohr Diagram - also describes How to Do Bohr Diagrams and labeled as: how to decrystallize honey,how to i renew my passport,how to r hop a barrel,how to screenshot on mac,how to z drop smash 4, with resolution 1262px x 1633px Atomic # atomic mass # of . Bohr model drawing (25 points). 2 from Identify the number of protons, neutrons, and electrons given a nuclide symbol. Not only do isotopes differ in mass, but some may be radioactive. Draw a bohr model of the following atoms and answer the questions about each one. Bohr model drawing (25 points).
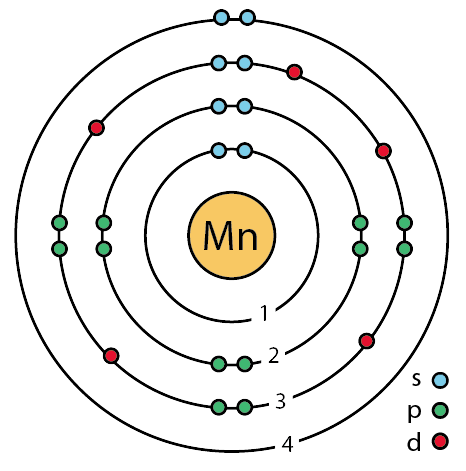
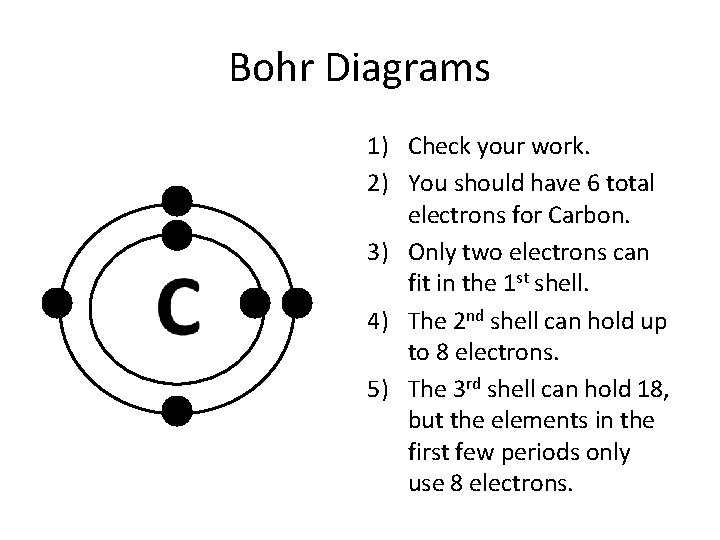
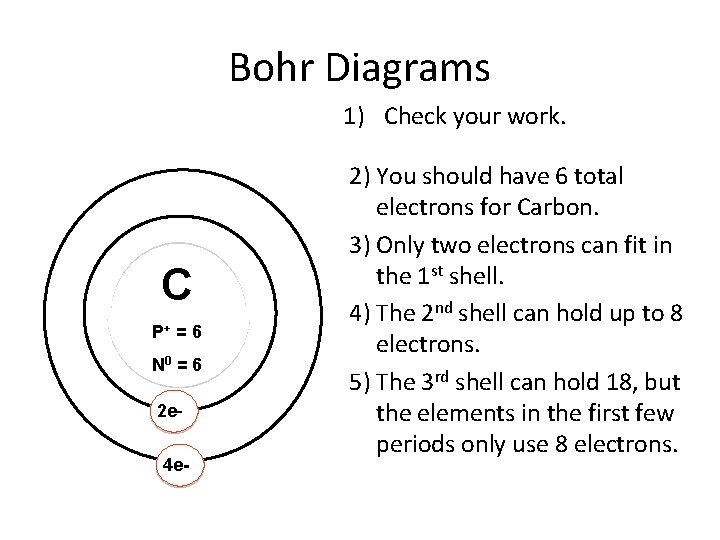
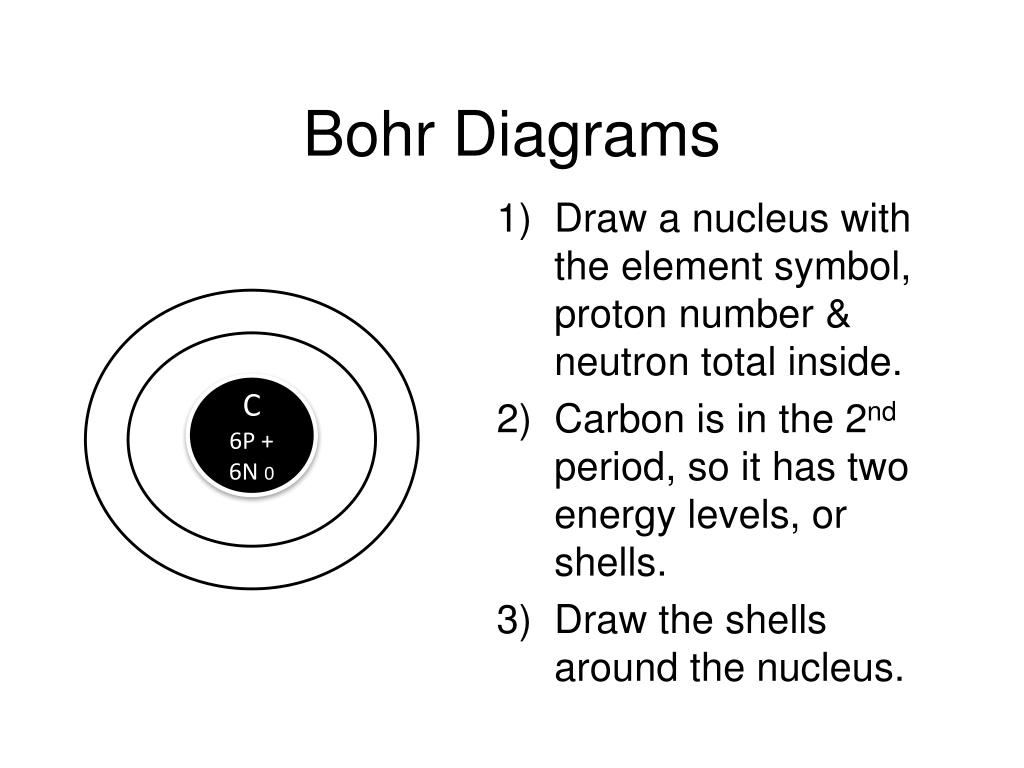
Bohr-Rutherford Diagrams We have looked at atomic models and the structure of atoms. Today we will practice drawing those models for the elements on the periodic table. Remember that protons and neutrons are found in the nucleus of the atom and the electrons are found outside the nucleus. Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ... Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He How to draw the Bohr-Rutherford Diagram for Germanium. The order of filling makes Bohr-Rutherford Diagrams for Elements beyond #20 (Calcium) tough. 2 in th...
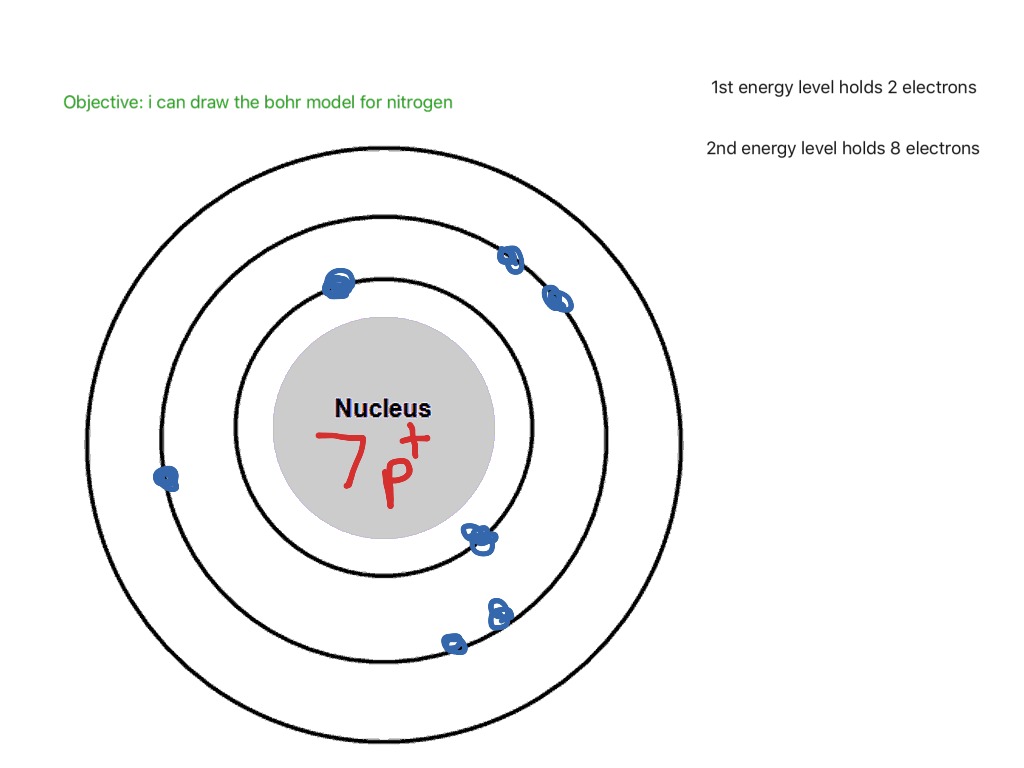
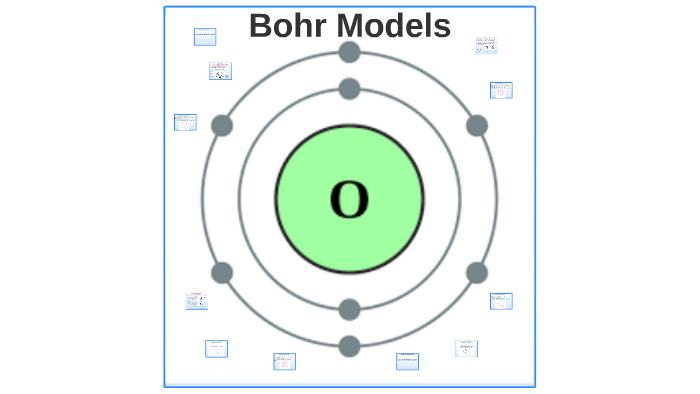
Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ... Rules for Drawing Bohr-Rutherford Diagrams Bohr's model of the atom can be combined with Rutherford's model in diagrams that summarize the numbers and positions of all three subatomic particles. For example, consider the following diagram for Phosphorous: There are certain rules to follow when drawing these diagrams: How do you draw and label a Bohr model for O and P. how to draw bohr diagrams michelle bartels bohr diagrams 1 draw a nucleus with the number of protons and neutrons inside 2 carbon is in the 2nd period so it has two energy levels or shells. blank bohr model worksheet blank fill in for first 20 elements. draw bohr diagrams for covalent pounds ... Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. C 3) The first shell can only hold 2 electrons. 9. Bohr Diagrams 1) Since you have 2 electrons already drawn, you need to add 4 more. C 2) These go in the 2nd shell. 3) Add one at a time -starting on the right side and going counter clock-wise. 10.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Iodine Bohr Diagram. Iodine 2,8,18,18,7. I. Sulfur!! Sulfur!! Atom Model Project, Mass Number, Bohr Model, Teaching Chemistry, [Bohr Model of Iodine] Bohr Model, Atomic Number, School Stuff, Health. This Pin was discovered by Mi Escuelita Montessori Montessori. Discover (and save!) your own Pins on Pinterest.
HOW TO DRAW BOHR DIAGRAMS - In this video, I'll teach you how to draw bohr diagrams for carbon (C), sodium (Na), and phosphorous (P). The steps to drawing bo...
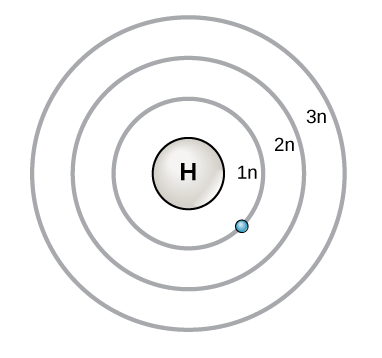
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom.
Bohr Diagrams 1) Since you have 2 electrons in the first shell, you need to add 4 more. C P+ = 6 N0 = 6 2) These go in the 2nd shell. 3) Again, write e- and the number of electrons. 2e- 4e-. 9. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon.
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons

Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels
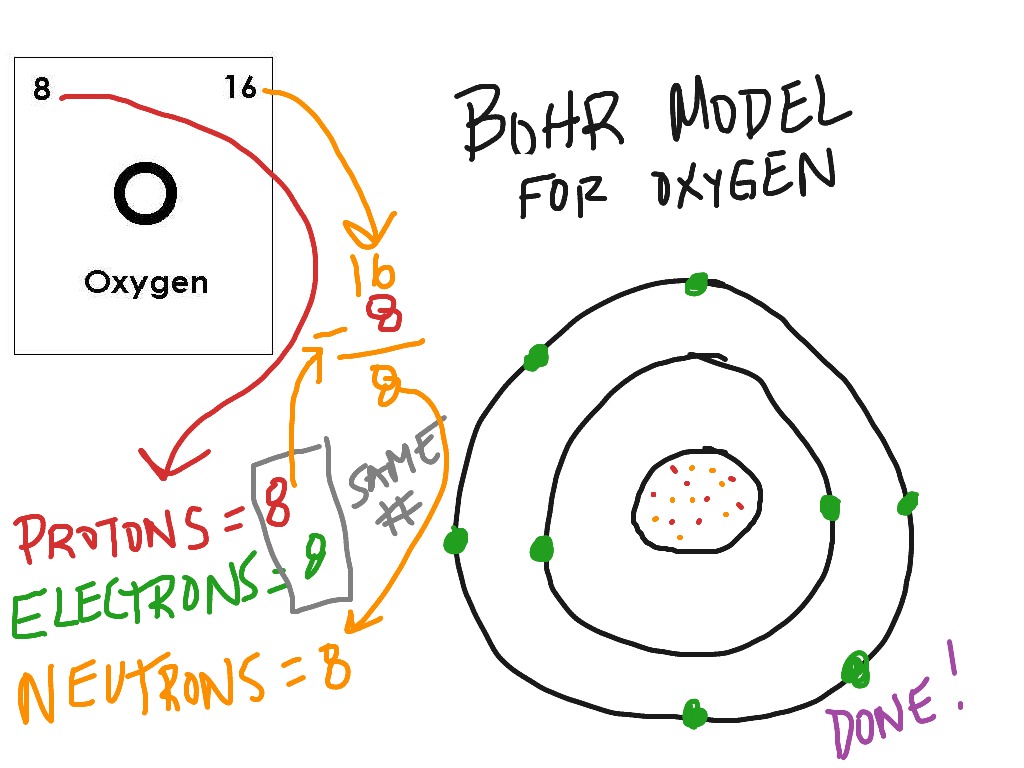
The Bohr model of Carbon is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains 4 electrons. Carbon is neutral and its atomic number is 6, hence, the number of protons and electrons available for its Bohr diagram is also 6. The number of neutrons for the Bohr diagram of Carbon can be found by ...
A step by step program for creating Bohr Diagrams and Electron dot structures. Usefule for Index Cards and Flash cards for chemistry and physical science stude… Slideshare uses cookies to improve functionality and performance, and to provide you with relevant advertising.
Bohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an atom, they can be useful to help with visualizing electrons orbiting a nucleus. Drawing Bohr-Rutherford diagrams is super easy using the following steps:
Drawing Bohr Models 3. Draw the first shell 4. Add the electrons, the first shell can only hold 2 P:9 N:10. Drawing Bohr Models 5. Draw the next shell if you have more electrons to add P:9 N:10. Drawing Bohr Models 6. Add your electrons, the 2nd shell can hold up to 8 Be sure to add them one at a time
Which Bohr Diagram Shown Above Represents The Purple Band Seen In The Hydrogen Emission Spectra Below The Red Band The Blue Band The Blue Green Band Explain How You Know Wyzant Ask
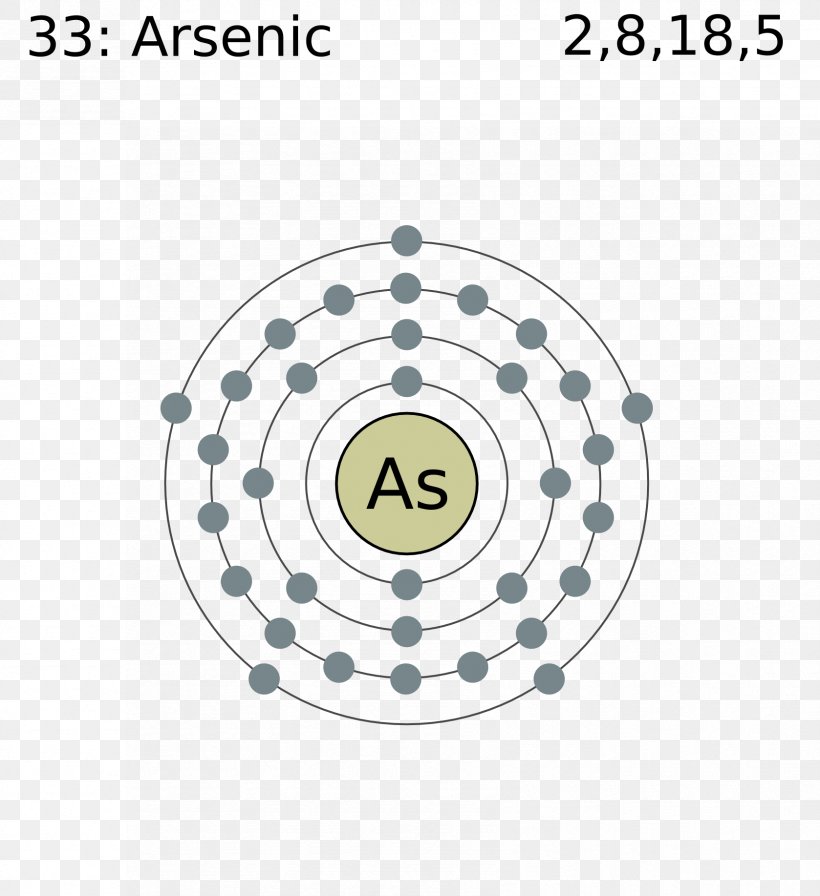
Bohr Model Atom Zirconium Bohr Radius Chemical Element Png 1678x1835px Bohr Model Area Atom Atomic Number

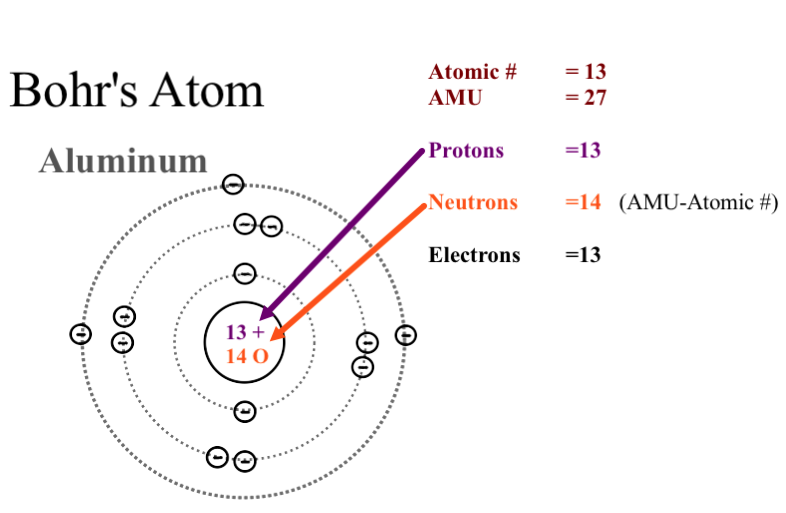
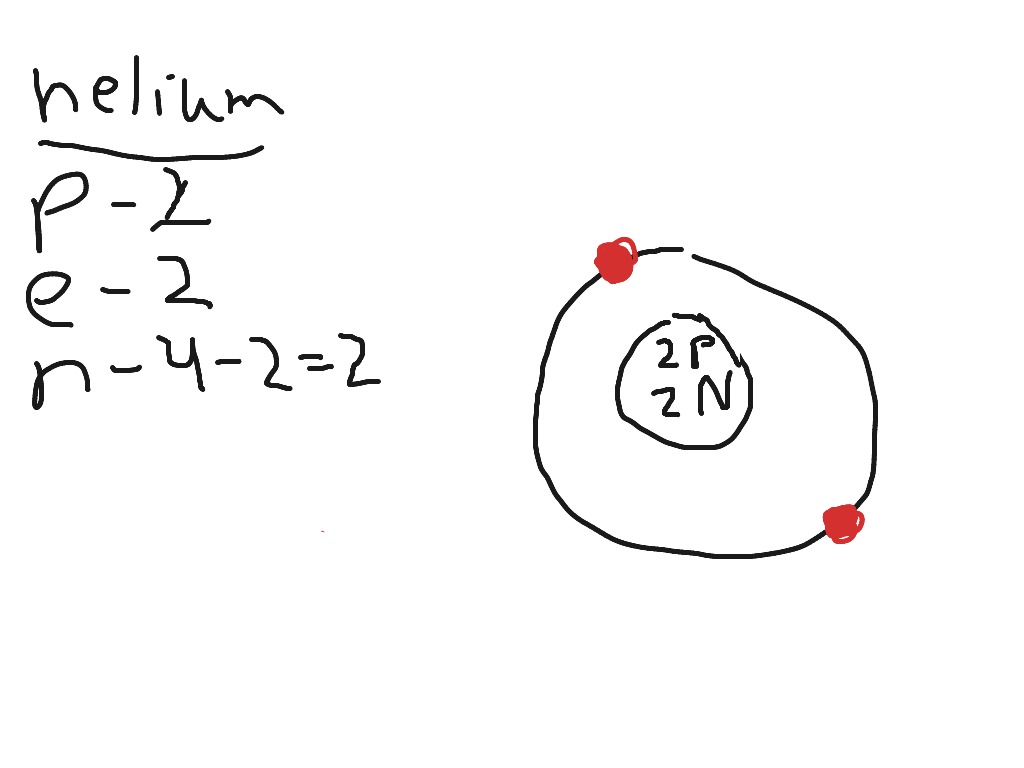
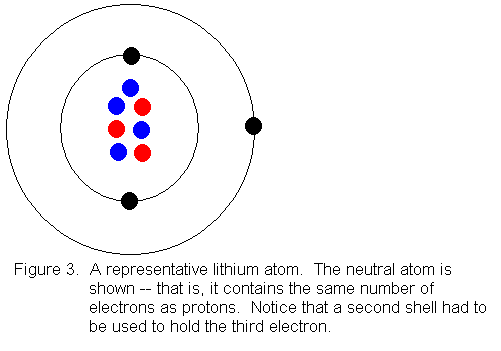













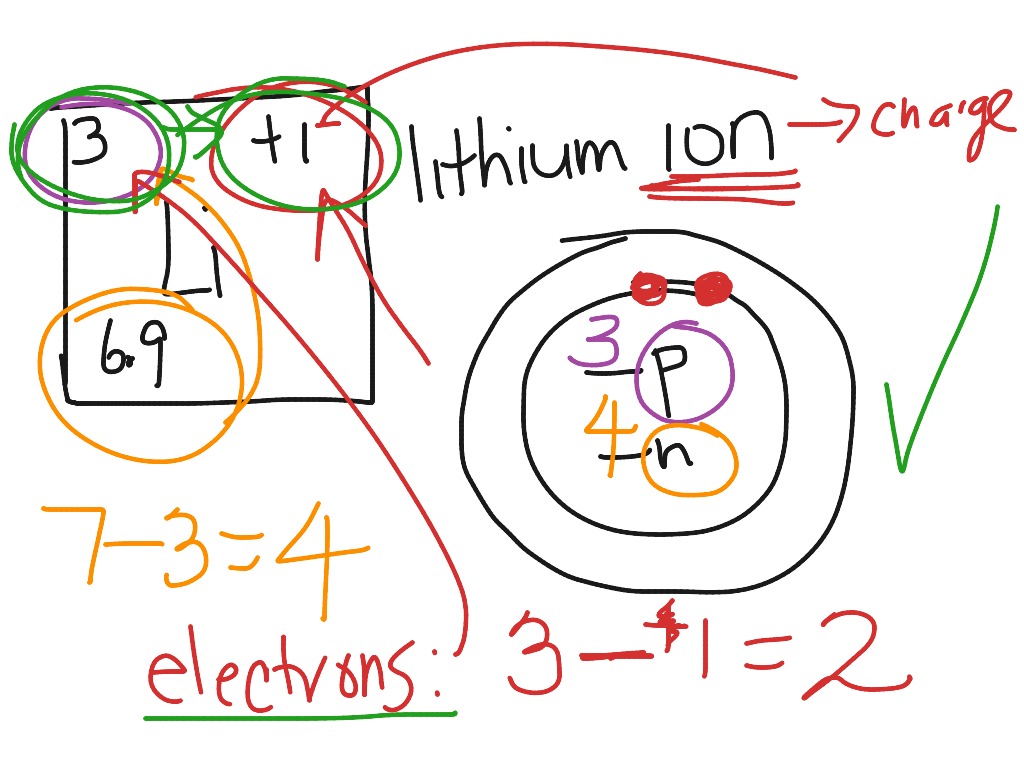
0 Response to "42 how to do a bohr diagram"
Post a Comment