36 endothermic reaction energy diagram
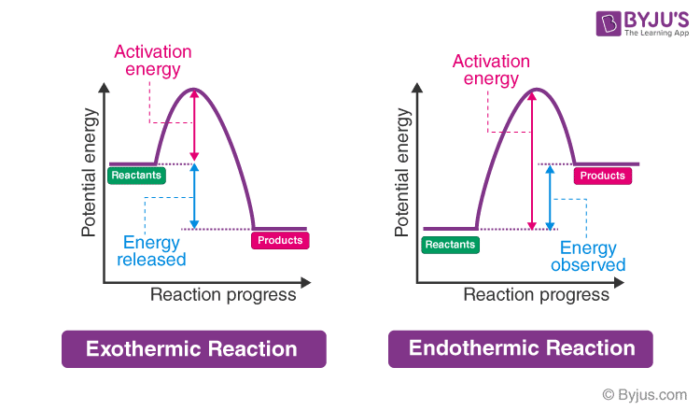
The energy level decreases in an exothermic reaction. This is because energy is given out to the surroundings. Graph of energy against progress of reaction.
Warning: This may be a long post, so bear with me. I currently have some doubts with Kinetics while going over my notes. Consider the following diagram: http://imgur.com/sgCWwFn 1. Why do we have that from the transition state (as it is a "combined" form of the reactants, shouldn't there be "bonds" as well?) we have a potential energy decrease by going to the products? Wasn't it that bond breaking was an endothermic process? If so, why is the case that the potential energy of the system is goi...
Jun 01, 2018 · Energy level diagram for an exothermic reaction is shown below. Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled.

Endothermic reaction energy diagram
01.06.2020 · Energy diagrams show the energy levels of reactants and products in a reaction. General energy diagrams for exothermic and endothermic reactions (©2020 Let’s Talk Science). You can see from the diagram above that the energy level of the products of an exothermic reaction is lower than the energy level of the reactants.
When you draw a Potential Energy Diagrams, you can see the activation energy and if the reaction is exo/endothermic. But some books when explaining the activation energy uses Gibbs only for the y-axis (not potensial), and then you can see if its spontanous or not. The activation energy is some sort of Gibbs isnt it? usually written as Ea or G**? If you are discussing reaction rates is there any difference which diagram you use?
ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages
Endothermic reaction energy diagram.
Consider, for example, a diagram that charts the energy change when a candle burns. Wax (C 34 H 70) combusts in the presence of oxygen (O 2) to yield carbon dioxide (CO 2) and water (H 2 O). Because more energy is released when the products are formed than is used to break up the reactants, this reaction is exothermic. Brittny All of this stuff relates to thermodynamics—the …
52 Sketch the potential energy diagram for an endothermic chemical reaction that shows the activation energy and the potential energy of the reactants and the potential energy of the products. Answer--> 1/04. 16 Which statement best explains the role of a catalyst in a chemical reaction? (1) A catalyst is added as an additional reactant and is consumed but not …
An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured …
Sep 02, 2021 · Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
03.08.2016 · This is asking you to draw a potential energy diagram for an endothermic reaction. Recall that DeltaH_"rxn", the enthalpy of reaction, is positive for endothermic reactions, i.e. the product(s) (right) are higher in energy than the reactant(s) (left) and energy was absorbed. (Energy increases from bottom to top.) Since... the activation energy for the …
The general equation for an endothermic reaction is: Reactants + Energy → Products. How can u tell if a reaction is exothermic? When a chemical reaction happens, energy is transferred to or from the surroundings. When energy is transferred to the surroundings, this is called an exothermic reaction, and the temperature of the surroundings increases. Examples of …
Aug 22, 2020 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
• In an Exhothermic reaction, the energy constantly being released by the reaction allows the process to continue once it has been started; but, in an Endothermic reaction, the process has to suck energy in continuously from the surrounding atmosphere – and by doing so, THAT is what enables it to keep going! If you were to place the liquid reactants in an Endothermic reaction …
hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic...
1. If 6.0 grams of NaCl is dissolved in enough water to make a 300.0-ml solution, calculate the w/v percentage of NaCl. 2. What is the molarity of a solution made from 15.5 grams of NaCl dissolved in enough water to make 1.50 liters of solution? 3. How many grams of HNO3 are there in 75.0 ml of 1.25 M HNO3 solution? 4. What is the concentration of a solution made from 23.5 ml of 8.95 M HCl diluted to 95.0 ml? 5. Which solution will have the lowest freezing point: 1 M NaCl, 1 M M...
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in …
Exothermic reactions uses/loses energy/heat during the reaction, so when drawing the energy diagram, you want the reactants on the graph to be higher than the ...
MUST KNOW INFORMATION, Test takes place April 28, 7pm pst should be about 30 min long so im Willing to pay 30 bucks ​ Know the signs that a chemical reaction has occurred. Know how to write a balanced chemical reaction. Know how to write a total ionic chemical reaction and a net ionic chemical reaction. Know the strong acids and the strong bases (these break up in aqueous solutions).Know how to use the solubility Rules to determine if salts are soluble or insoluble. Know the di...
I am confused on whether in a reaction coordinate diagram, does the difference between the reactants and products equal deltaH or deltaG I have seen graphs that demonstrate both. If some could explain that would be great!   for reference this khan article states it as deltaH: https://www.khanacademy.org/test-prep/mcat/chemical-processes/thermochemistry/a/endothermic-vs-exothermic-reactions   This khan article states it as deltaG: https://www.khanacademy.org/science/biolog...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below.
I'm tutoring someone who is struggling with topics from content category 5E. I already recommended Leah4Sci and they are already using Jack Westin. What other resources did you like for learning these topics besides simply reading a review book? ​ ***Content Category 5E: Principles of chemical thermodynamics and kinetics*** **Enzymes (BC, BIO)** * [Classification by reaction type](https://jackwestin.com/resources/mcat-content/enzymes/classification-by-reaction-type) * [Mechanism]...
Hello everyone, I'm a sec 5 student who just finished O Level and currently waiting for poly to start. Recently, I created my own O Level Science paper by hand and welcome anyone *(Teachers, Students and others)* to check my paper for errors *(Questions and grammatical)* and provide me your suggested answers and solutions *(Can be on paper or in word)* . Also, I accept any feedbacks *(Positive or negative)* on ways that I can improve my exam papers. Moreover, I allow anyone to use my questio...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
I've been having trouble with this particular set of questions, I can't tell if I just don't grasp the concepts, or if I actually do know the answers and just can't put it together. 34. Assume that the following reaction is a single step reaction in which a C−Br bond is broken as the C−I bond is formed. The heat of reaction is +38 kJ/mol. I−(aq) + CH3Br(aq) + 38 kJ → CH3I(aq) + Br−(aq)CBrHHHCIHHH a. With reference to collision theory, describe the general process that takes place as this reac...
9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
When looking at an energy profile diagram for an endothermic reaction, the energy state of the reactants is lower compared to that of the products. So shouldn't that mean that reactants are the 'preffered' state? Why does for example ammonium nitrate dissolved in water happen spontaneously? Am I understanding 'lowest energy state' = 'most stable state' incorrectly?
Jul 21, 2021 · 10+ Endothermic Energy Diagram. Endothermic reactions often produce a decrease in temperature. Phase diagrams give the relationship among the solid, liquid and vapor phase of a compound. Siyavula's open physical sciences grade 11 textbook, chapter 12 on energy and chemical change covering exothermic and endothermic reactions. The activation energy is the energy that must be provided to the reactants so that they can overcome the.





























0 Response to "36 endothermic reaction energy diagram"
Post a Comment