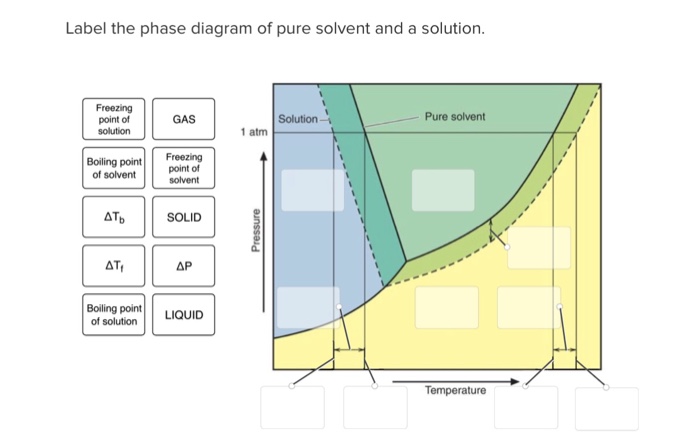
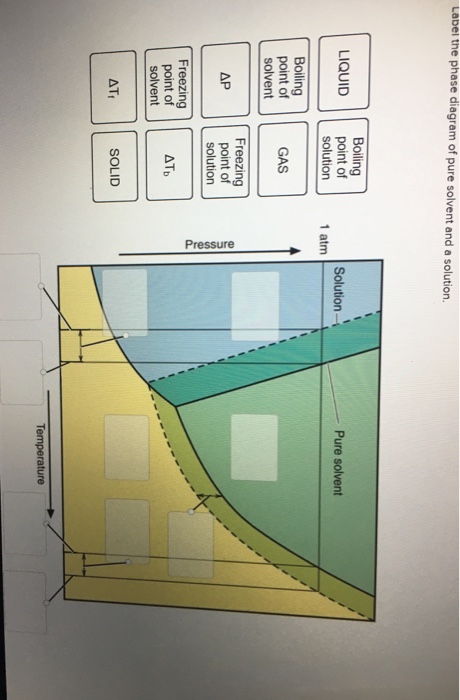
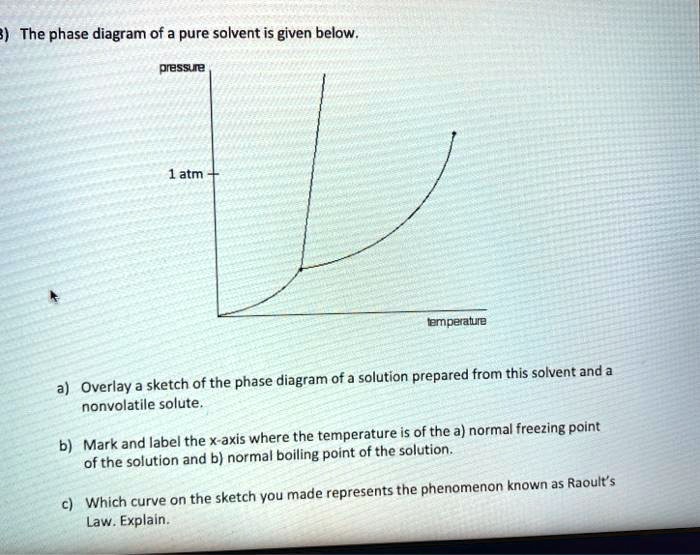
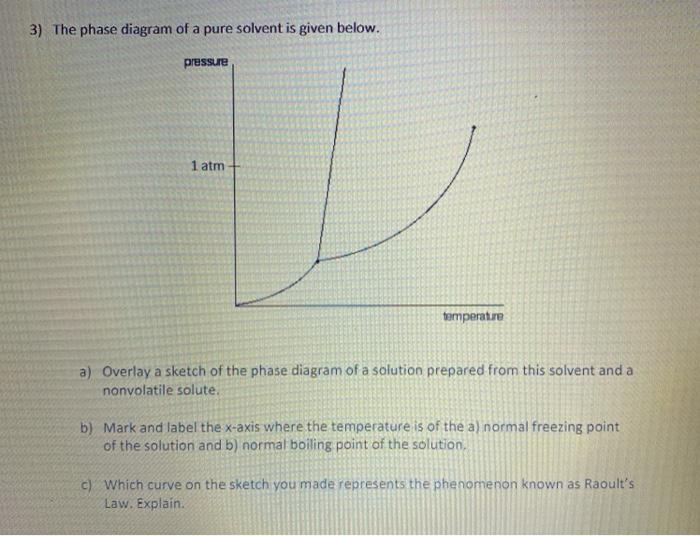
36 label the phase diagram of pure solvent and a solution.
Label the phase diagram of pure solvent and a solution. Introduced to the system forming a homogeneous solution however the phase diagram drastically changes. Phase solid argon must be the denser phase. E the temperature of the substance is increasing. A pure solvent solution 0 time 0 5 10 ...
Label the phase diagram of pure solvent and a solution. Skilled solution 100 25 rankings this downside has been solved. You can think of this as a simple phase diagram. I came across a situation in which my chemistry textbook gives phase diagram of a pure solvent and a solution with the same ...
= Final stage. This is where the fresh solvent S enters the system and the final raffinate leaves the system. = Composition of the mixture representing the overall system. Points (and ) and (and ) must be connected by a straight line that passes through point . will be located within the ternary phase diagram. = Operating point.
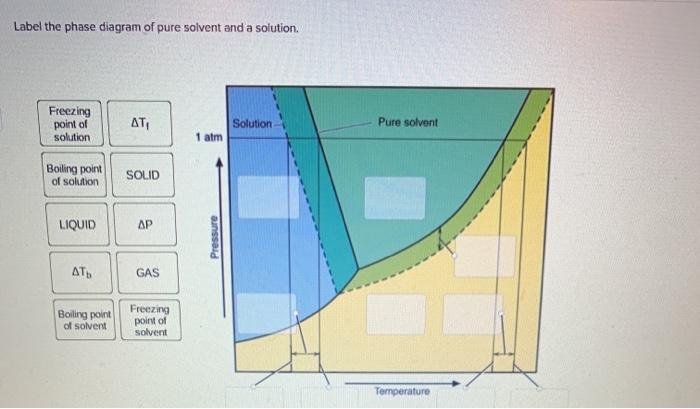
Label the phase diagram of pure solvent and a solution.
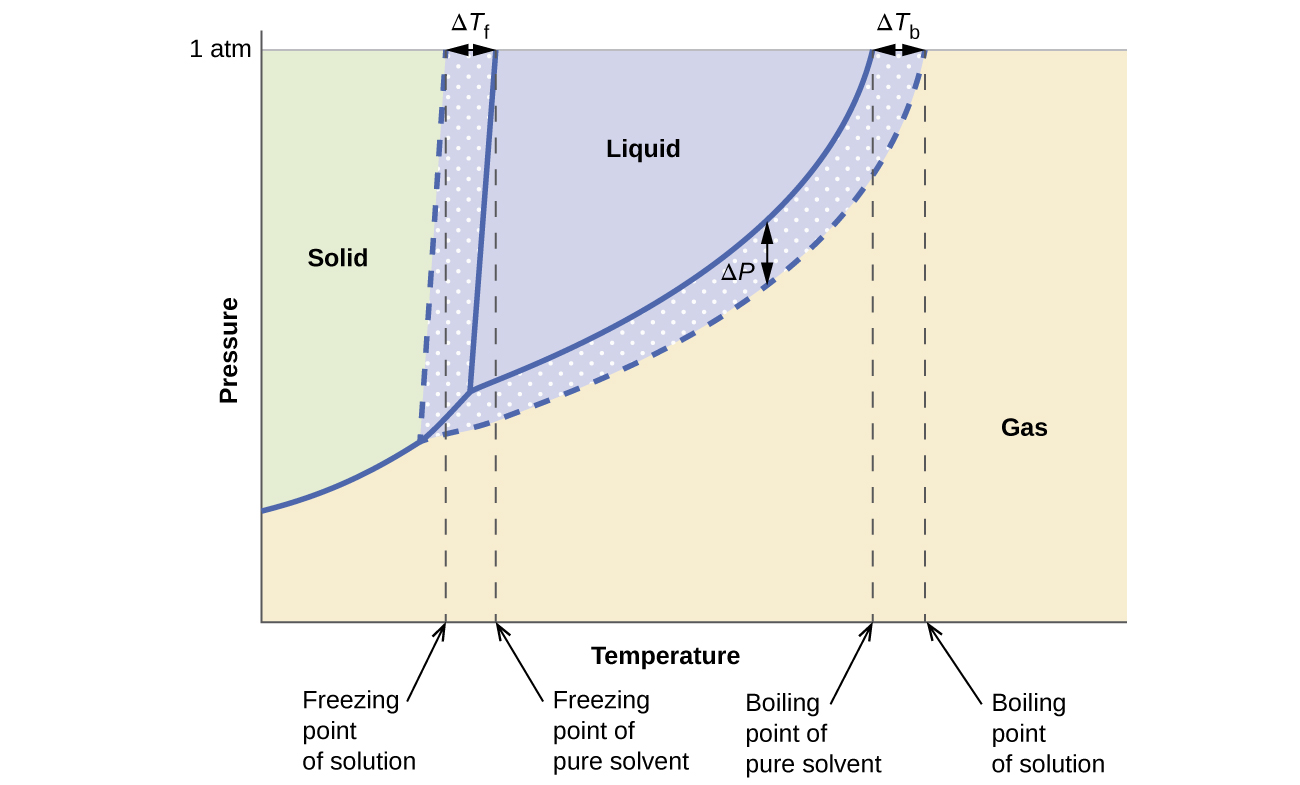
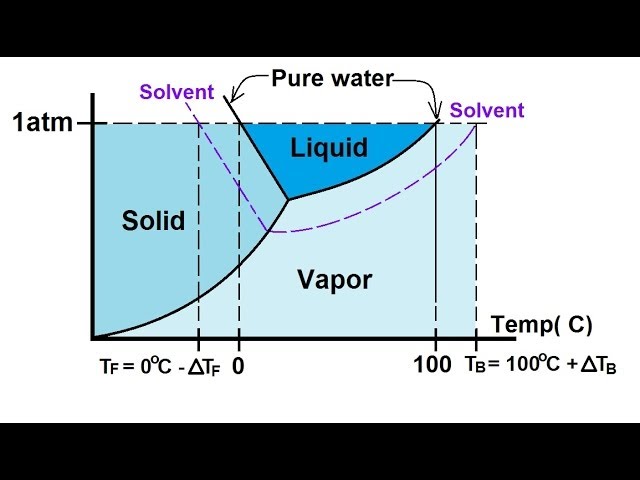
February 17, 2021 - The effect of Raoult's Law is that the saturated vapor pressure of a solution is going to be lower than that of the pure solvent at any particular temperature. That has important effects on the phase diagram of the solvent. The next diagram shows the phase diagram for pure water in the region ...
Phase diagrams for polystyrene solutions in poor solvents and theta-solvents have been determined as functions of concentration, molecular weight and polydispersity, pressure, temperature, and H/D...
Label the phase diagram of pure solvent and a solution. If you have a mixture of 100 g of potassium nitrate and 100 g of water and the temperature is above 57c you have a single phase a solution of potassium nitrate. Diagram of pure solvent and a solution display transcribed symbol text label the section diagram of pure. Starting from the phase diagram for a pure volatile solvent how is the ...
Label the phase diagram of pure solvent and a solution..
The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of ... Use the data above to draw a phase diagram for argon. Label the axes and label the regions in which the solid, ... phase, solid argon must be the denser phase. (K) 5. (a) pure solvent solution 0 Time 0 5 10 15 20
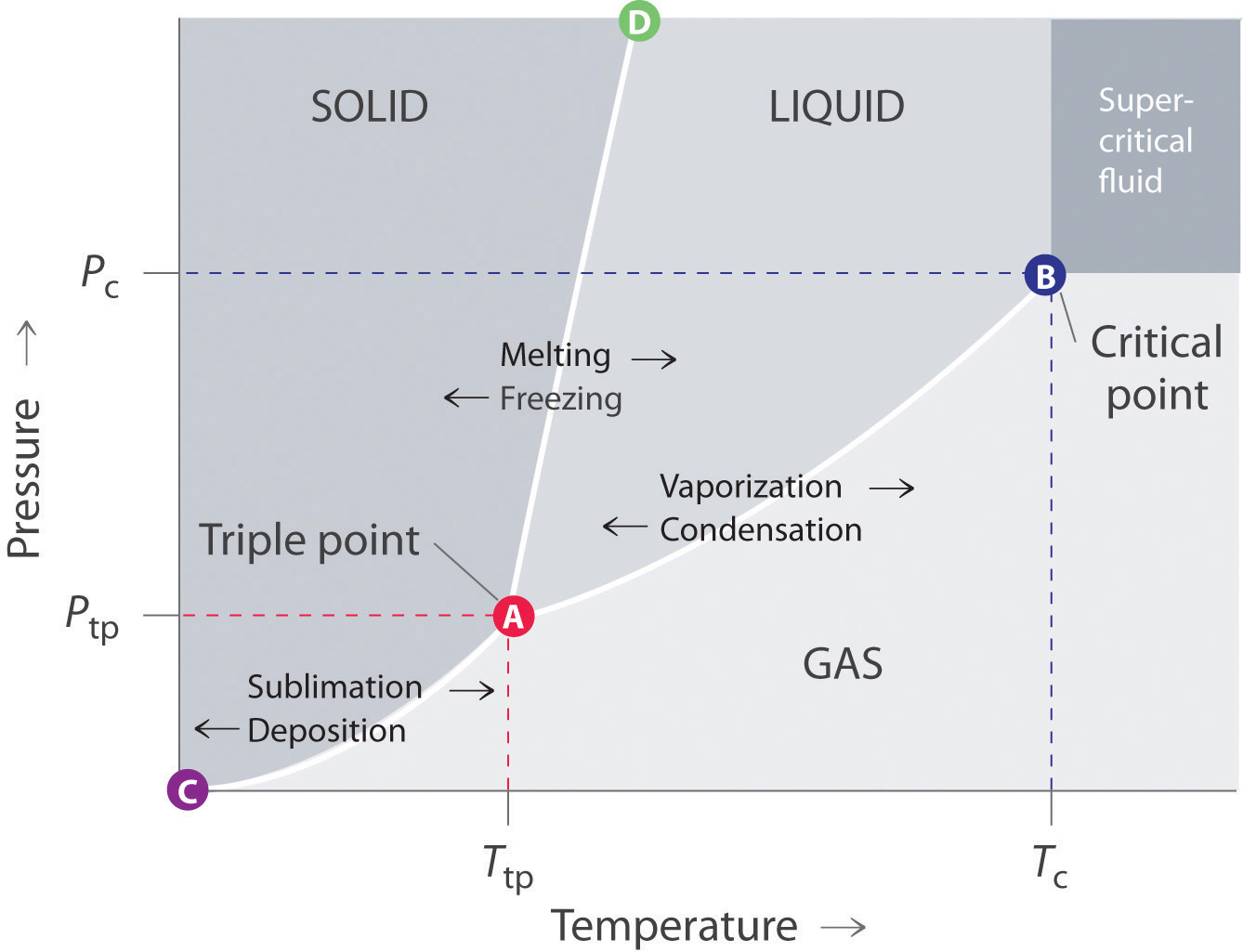
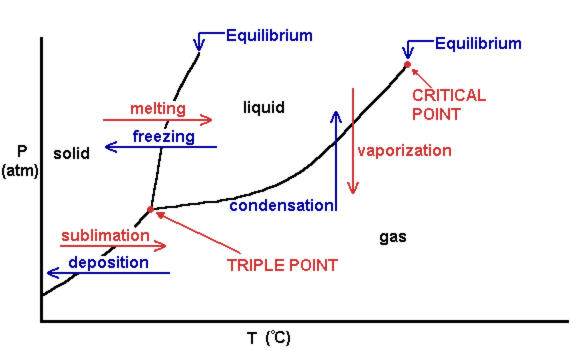
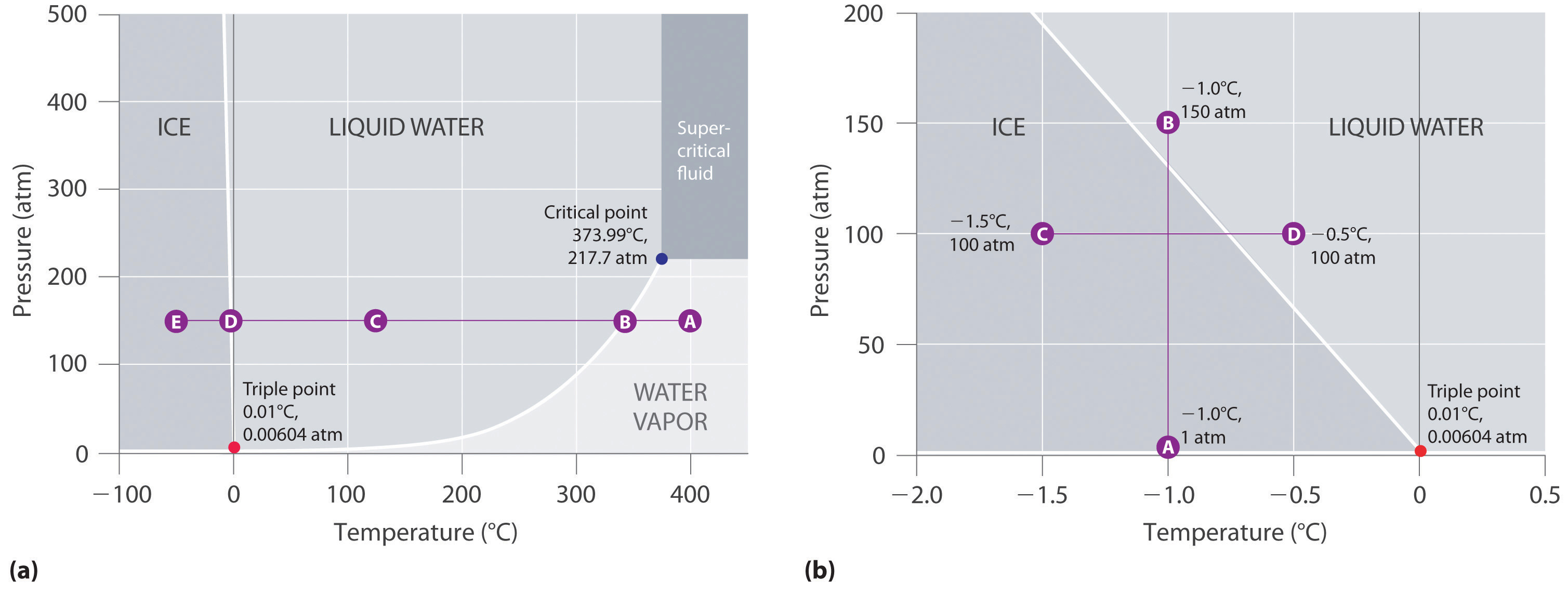
A phase diagram is a temperature-pressure plot that summarizes the conditions under which a substance exists as a solid, liquid, or gas. The curves that separate the phases are known as phase boundaries. Each curve represents the equilibrium between the phases on either side of the curve.
When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution) can disrupt important interactions between solvent molecules, changing the temperature at which the solvent would typically freeze or boil.
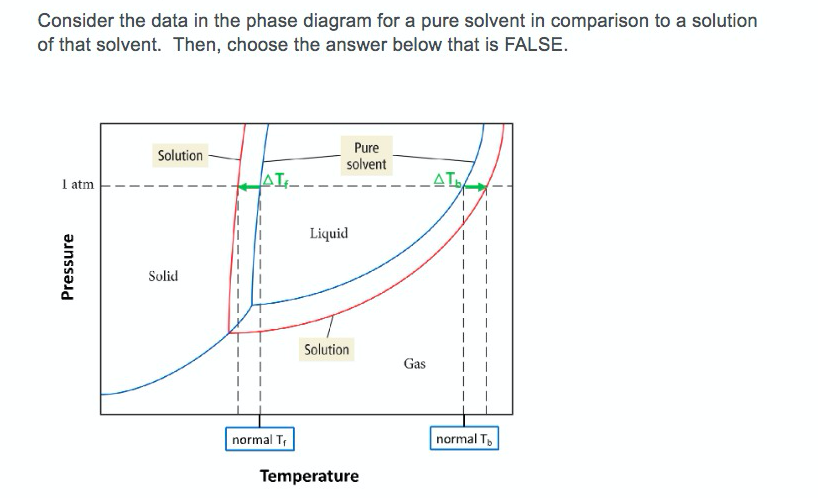
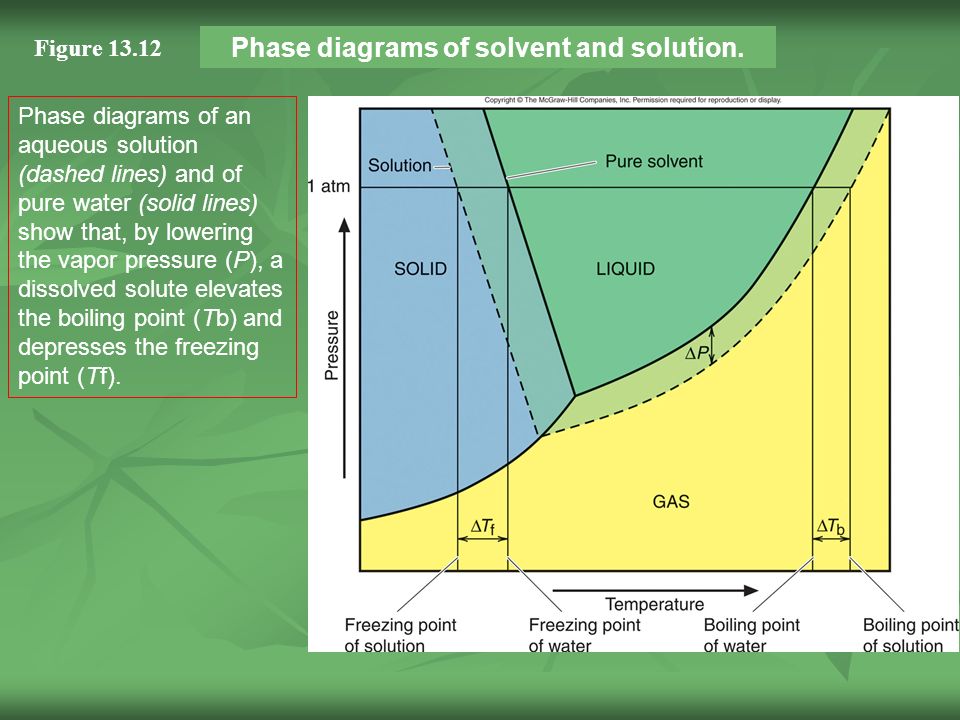
February 28, 2021 - Thus the boiling point of a solution is always greater than that of the pure solvent. We can see why this must be true by comparing the phase diagram for an aqueous solution with the phase diagram for pure water (Figure \(\PageIndex{4}\)). The vapor pressure of the solution is less than that ...
Portions of the ternary phase diagram were determined by titrating a PEO solution in the polar solvent with heptane under visual inspection, in a temperature controlled environment (within 1 degree for experiments at - 50ºC and within 0.1 degree for the other temperatures).
Label the phase diagram of pure solvent and a solution. Phase diagrams of pure water and an aqueous solution of a nonvolatile solute. It has b atom as the solute component. Pure a and pure b are also considered to be α and β phases respectively. Shown above is a phase diagram for water. Expert answer 100 35 ratings. But why should vapor pressure lowering cause a decrease in the freezing ...
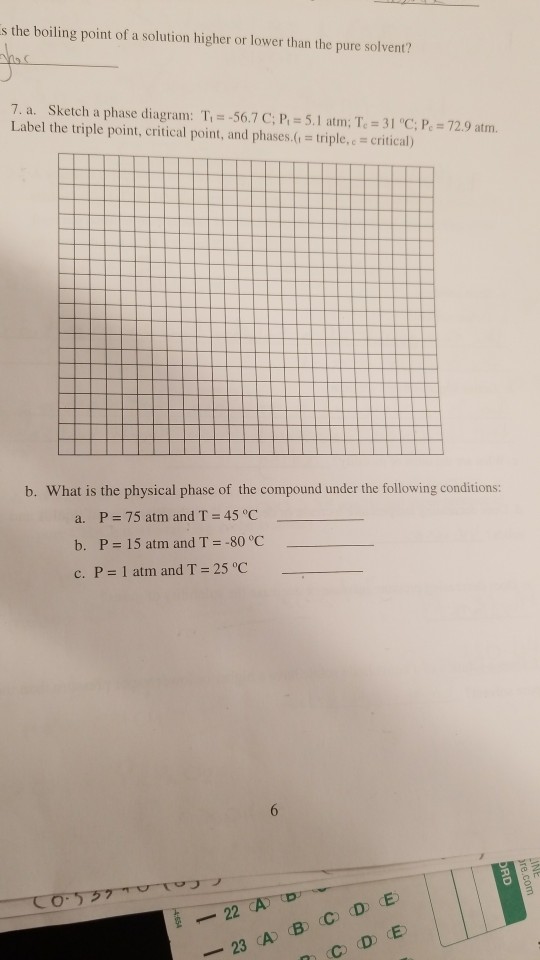
Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
50+ Ideas Label The Phase Diagram Of Pure Solvent And A Solution. Design and evaluation of multiple the evaporation of water from an aqueous solution is widely used in the food, desalination, pulp, and chemical industries. Usually, a large amount of energy is consumed in.
The figure shows the phase diagram of a system in which the liquids become fully miscible before they boil. Distillation of a mixture at a 1 leads to vapor with composition b 1, which condenses to completely miscible solution at b 2. Phase separation only occurs when the distillate is cooled to a point in the two-phase region such as point b 3.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. This is …. View the full answer. Transcribed image text: Label the phase diagram of pure solvent and a solution.
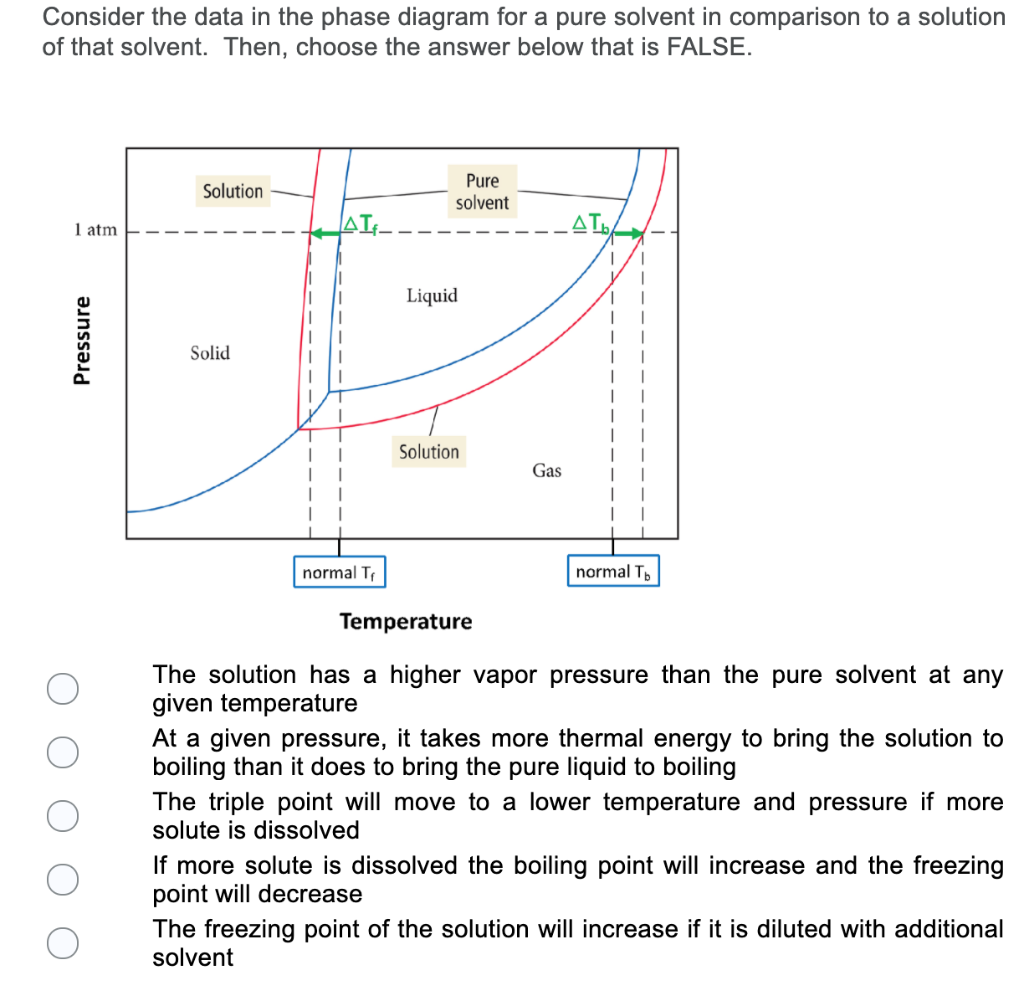
The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by L in t
A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
Label the phase diagram of pure solvent and a solution. The vaporization curve for the solution lies below the curve for pure water at all temperatures which results in an increase in the boiling point and a decrease in the freezing point of the solution. This is the case for many solutions comprising liquid solvents and nonvolatile solutes.
7 In Figure 9.3, the raffinate phase consisting of the solute A and the diluent D is fed into the system from the right hand side (RHS), with the total flow rate of o R (or R) and the concen- tration (in mole or mass fraction of the solute) of o x; note that the flow rate of just the diluent is D F The extract phase consisting of pure solvent S or the solvent S with some amount of solute
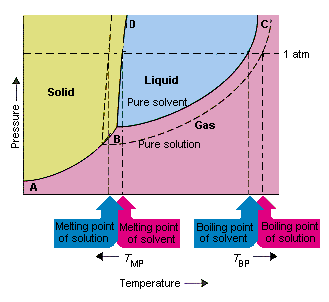
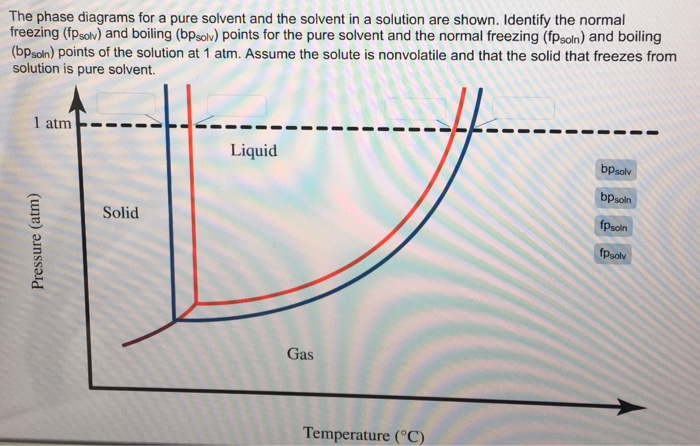
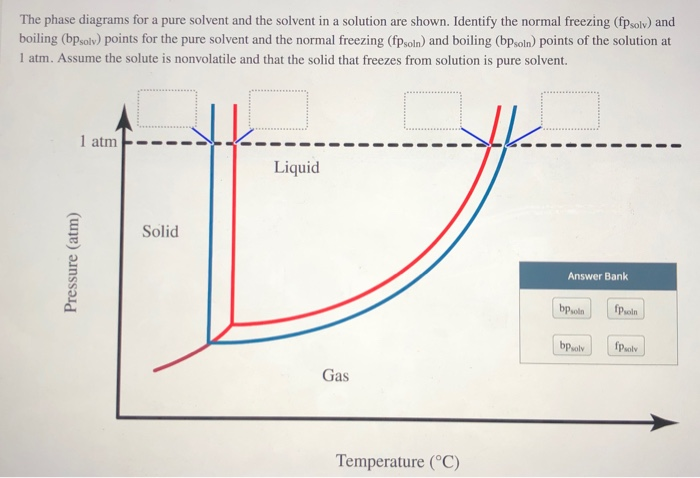
Transcribed image text: The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fp_solv) and boiling (bp_solv) points for the pure solvent and the normal freezing (fp_soln) and boiling (bp_soln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent.
Expt. 5: Binary Phase Diagram CHEM 366 V-3 Combination1,2,3 of equations (2), (3), (6), (7) and (10) leads to the equation T≅TA+ RTA 2 ΔHA lnXA (11) or T≅TA+ RTA 2 ΔHA ln(1−XB) (12) or T≅TA- RTA 2 ΔHA lnXB (13a) T≅TB- RTB 2 ΔHB lnXA (13b) where T and TA are freezing points (K) of the mixture II and pure A, R is 8.314 J/K mole, ΔHA is the molar enthalpy of fusion of A and XB ...
Because of the changes to the phase diagram, you can see that: the boiling point of the solvent in a solution is higher than that of the pure solvent; the freezing point (melting point) of the solvent in a solution is lower than that of the pure solvent.
the pure solvent is present. Thus the vapor pressure above the solution containing solute will be lower than the vapor pressure of the pure solvent. The dissolved solute molecules physically block the surface of the solvent, preventing some of the solvent molecules from evaporating at a given temperature. Shown below are two phase diagrams for ...
solution which is just the sum of the two Raoult's law vapor pressures (the sum of two straight lines is a straight line). Vapor Pressure Diagrams and Boiling Diagrams We are now ready to begin talking about phase diagrams involving two components. Our first few phase diagrams will involve only the liquid and gas (or vapor) phases.
Phase Diagram 1. Chapter-5 PHASE AND PHASE EQUILIBRIUM Prepared By: PALLAV RADIA Asst prof. AITS, RAJKOT. 2. Introduction: One of the most important objective of engineering metallurgy is to determine properties of material. The properties of material is a function of the microstructure which depend on the overall composition and variable such as pressure and temperature. Hence to determine ...
The figure below shows the consequences of the fact that solutes lower the vapor pressure of a solvent. The solid line connecting points B and C in this phase diagram contains the combinations of temperature and pressure at which the pure solvent and its vapor are in equilibrium.
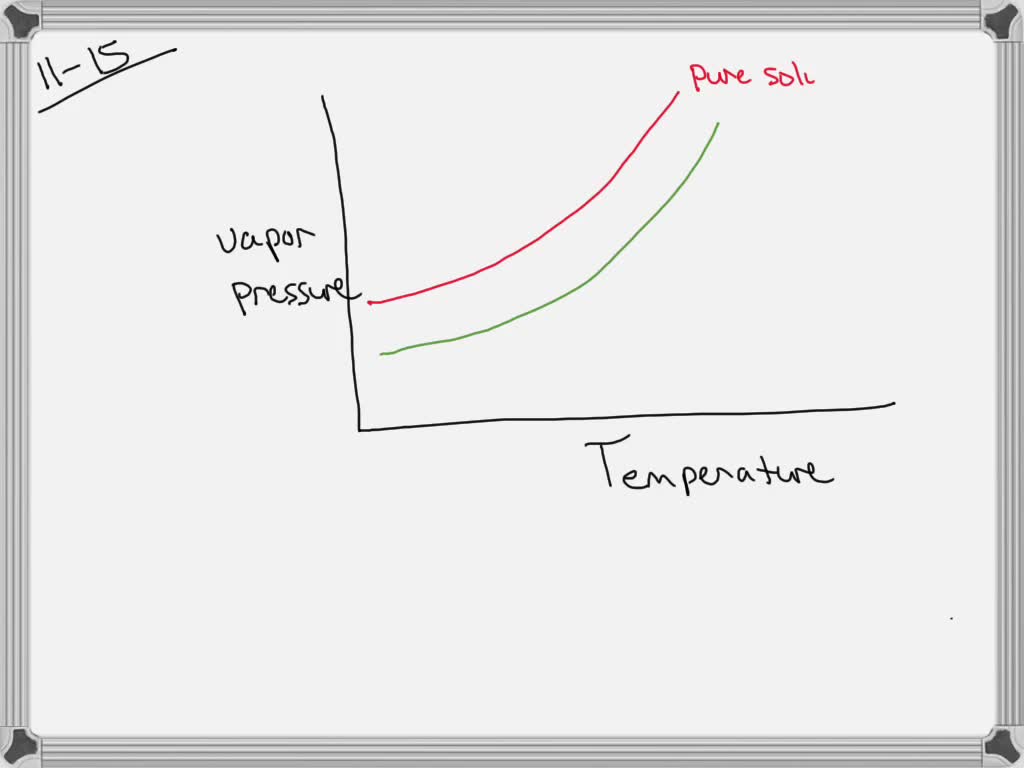
This portion of a phase diagram shows the vapor pressure curves of a volatile solvent and of a solution of that solvent containing a nonvolatile solute a which line represents the solution b what ar 2
The completely solidified alloy in the phase diagram shown is a solid solution because: Alloying element (Cu, solute) is completely dissolved in host metal (Ni, solvent) Each grain has same composition Atomic radius of Cu is 0.128nm & that of Ni is 0.125nm, Both elements are FCC; HRRs are obeyed. 12/3/2013 11:12 PM
Problem: Label the diagram of pure solvent and a solution. FREE Expert Solution Recall that a phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes.
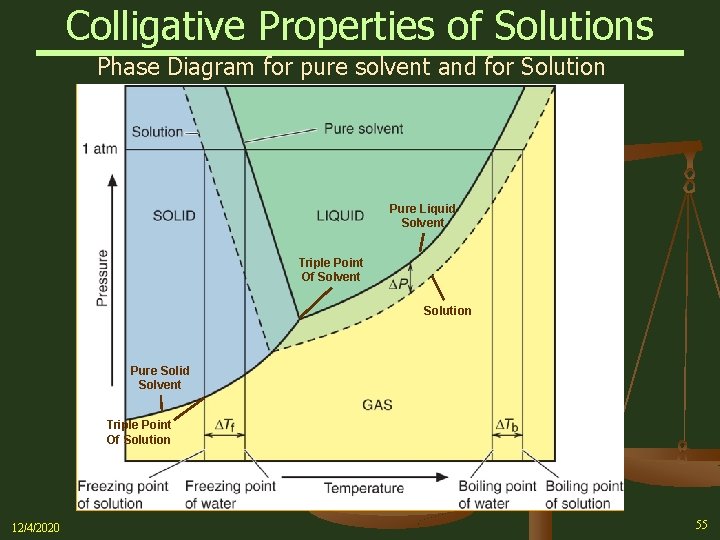
Phase diagrams for a pure solvent (solid curves) and a solution formed by dissolving nonvolatile solute in the solvent (dashed curves). The liquid-vapor curve for the solution is located beneath the corresponding curve for the solvent, depicting the vapor pressure lowering , Δ P , that results from the dissolution of nonvolatile solute.
Label the phase diagram of pure solvent and a solution. The α phase is a solid solution rich in a atom. For example the addition of a solute to a pure solvent making a solution can disrupt important interactions between solvent molecules changing the temperature at which the solvent would typically freeze or boil. A terminal phase or terminal solution is one that exists in the extremes of ...
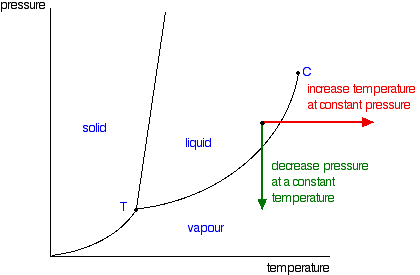
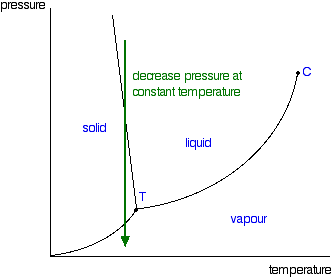
Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. Under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. At 2, it would be a liquid; and at 3, it would be a vapor (a gas).
September 21, 2018 - We study fluid-fluid equilibrium in the simplest model of ionic solutions where the solvent is explicitly included, i.e., a binary mixture consisting of a restricted primitive model (RPM) and neutral hard-spheres (RPM-HS mixture). First, using the collective variable method we find free energy, ...
January 31, 2018 - Export articles to Mendeley · Get article recommendations from ACS based on references in your Mendeley library
Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
Label the phase diagram of pure solvent and a solution. Shown above is a phase diagram for water. Well look now at the phase diagram for sodium chloride solution in some detail. Phase diagrams of pure water and an aqueous solution of a nonvolatile solute. Pure a and pure b are also considered to be α and β phases respectively. It has b atom as the solute component. A terminal phase or terminal solution is one that exists in the extremes of concentration 0 and 100 of the phase diagram.
Q. Label the diagram of pure solvent and a solution. Q. Consider this phase diagram for carbon. Which phases are present at the lower triple point?a) diamondb) graphitec) gasd) liquidWhich phase is stable...
Consider the following general phase diagram: Now, consider the pure substance, whose phase diagram was represented by the black curves. The normal freezing point of the pure substance at constant pressure is indicated by B, and its reduced normal freezing point (due to addition of nonvolatile solute to the pure liquid substance) is A, since Tdarr leftwards.
A phase diagram shows the conditions at which different phases are in thermodynamic equilibrium. Starting from the phase diagram for a pure volatile solvent how is the phase diagram change when a non volatile solute is dissolved in the solvent. The phase diagrams for a pure solvent and the solvent in a solution are shown. A volatile substance will readily. These phases are called terminal solid solutions.
where P A is the vapor pressure of component A of the solution (in this case the solvent), XA is the mole fraction of A in solution, and P A 0 is the vapor pressure of pure A. Equation 13.6.1 is known as Raoult's law, after the French chemist who developed it. If the solution contains only a single nonvolatile solute (B), then X A + X B = 1 ...
Isomorphous Phase Diagrams Phase diagram Indicate phases as a function of Temp., Comp. and Pressure (under equilibrium condition) Binary phase diagram A phase diagram for a system with two components. Isomorphous phase diagram A phase diagram in which the components display unlimited solid solubility. Chapter 10: Solid Solutions and Phase ...
Label the phase diagram of pure solvent and a solution. Skilled solution 100 25 rankings this downside has been solved. Chemistry 4 qs missed. You can think of this as a simple phase diagram. Starting from the phase diagram for a pure volatile solvent how is the phase diagram change when a non volatile solute is dissolved in the solvent. Show transcribed image text label the phase diagram of ...
11 Dec 2019 — Get the detailed answer: Label the phase diagram of a pure solvent and a solution.1 answer · Top answer: Explanation: A phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. ...
III.1. Draw phase diagrams for the following type systems. Label the regions and in-tersections of the diagrams, stating what materials (possibly compounds or azeotropes) are present and whether they are solid, liquid or gas. (a) One component, pressure-temperature diagram, liquid density greater than that of solid. (b) Two component,
Identify the solvent and solute in each of the solutions A) salt (NaCl) in water ... Also, assume that the engine coolant is pure ethylene glycol (HOCH2CH2OH), which is non-ionizing and non-volatile, and that the pressure remains constant at 1.00 atm. ... Label the phase diagram. A) Solid Phase B) Triple Point C) Sublimation Point/ Deposition Point
– Solutions – solid solutions, single phase – Mixtures – more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure ...












0 Response to "36 label the phase diagram of pure solvent and a solution."
Post a Comment