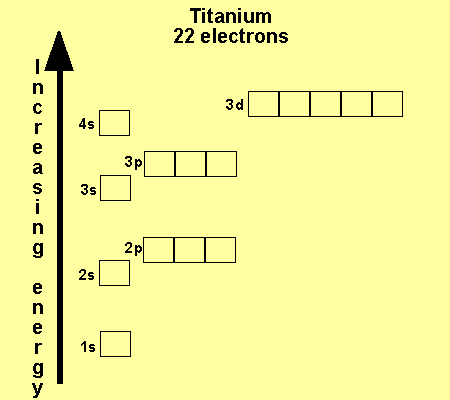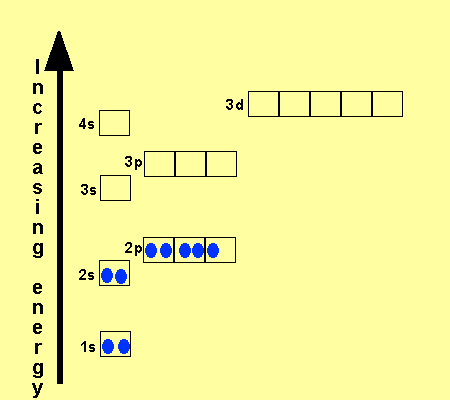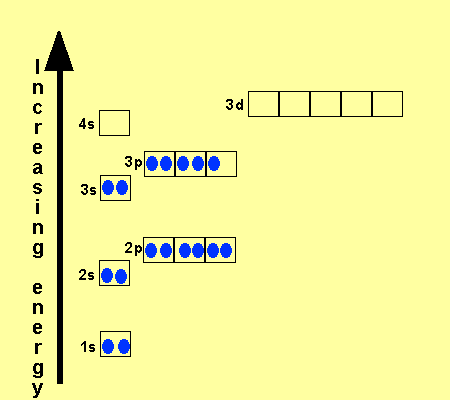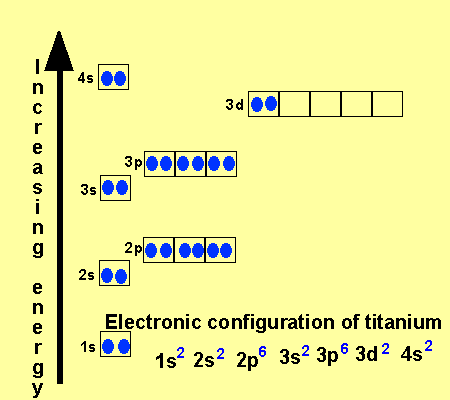
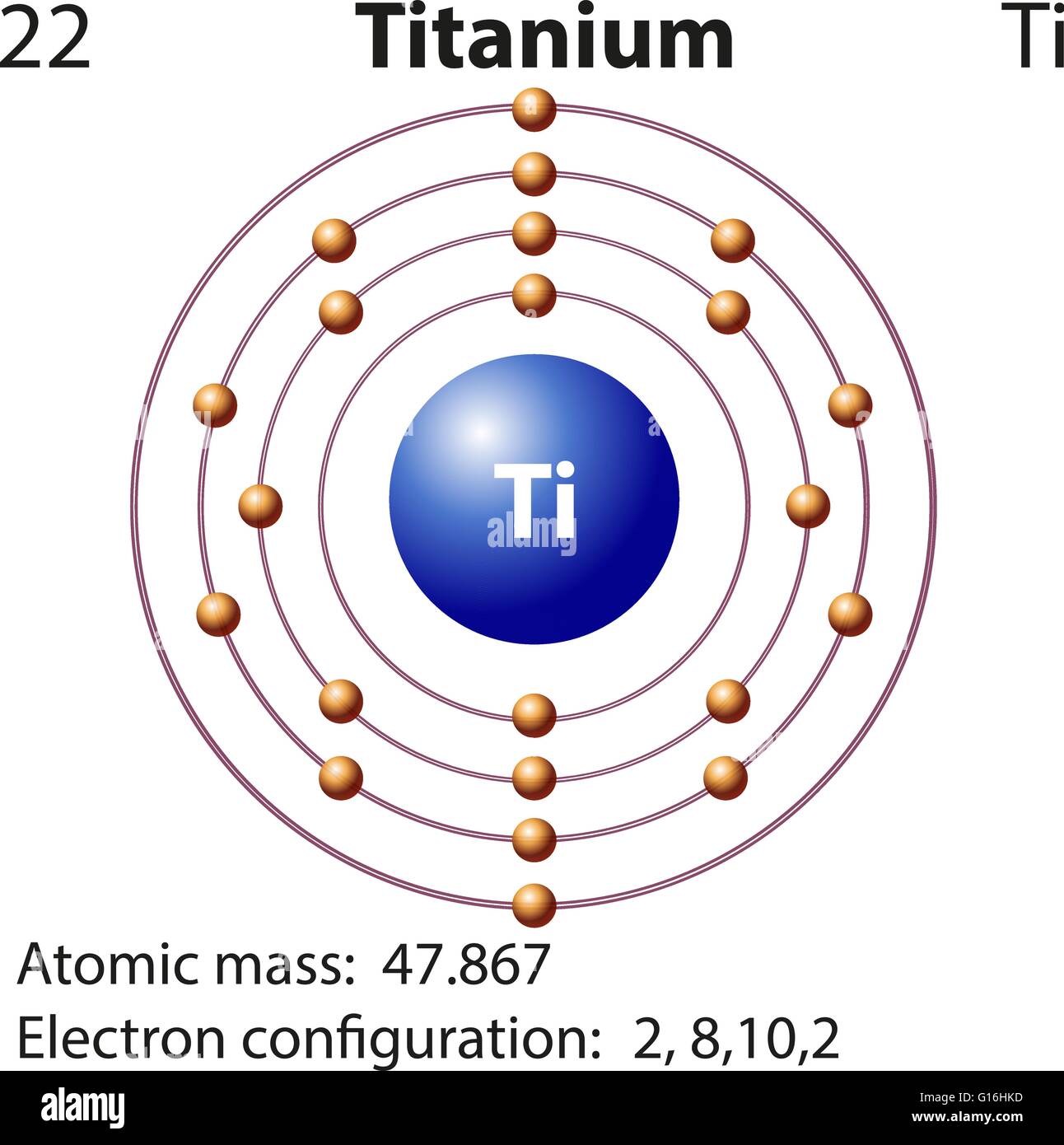
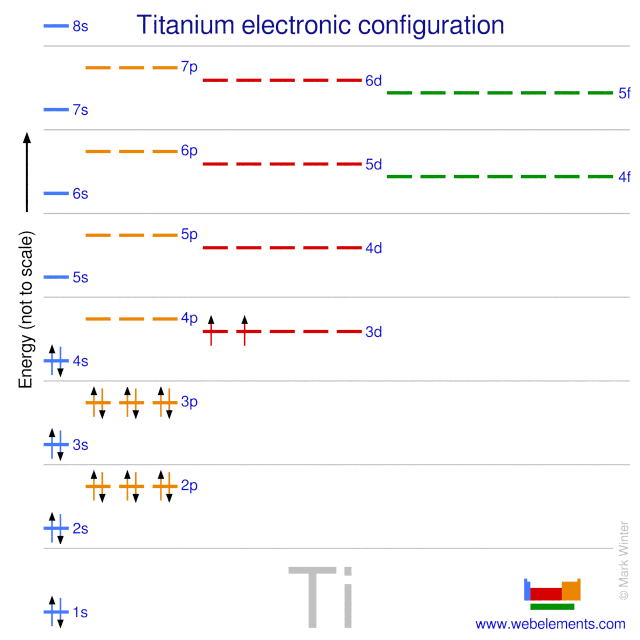
37 orbital diagram of titanium
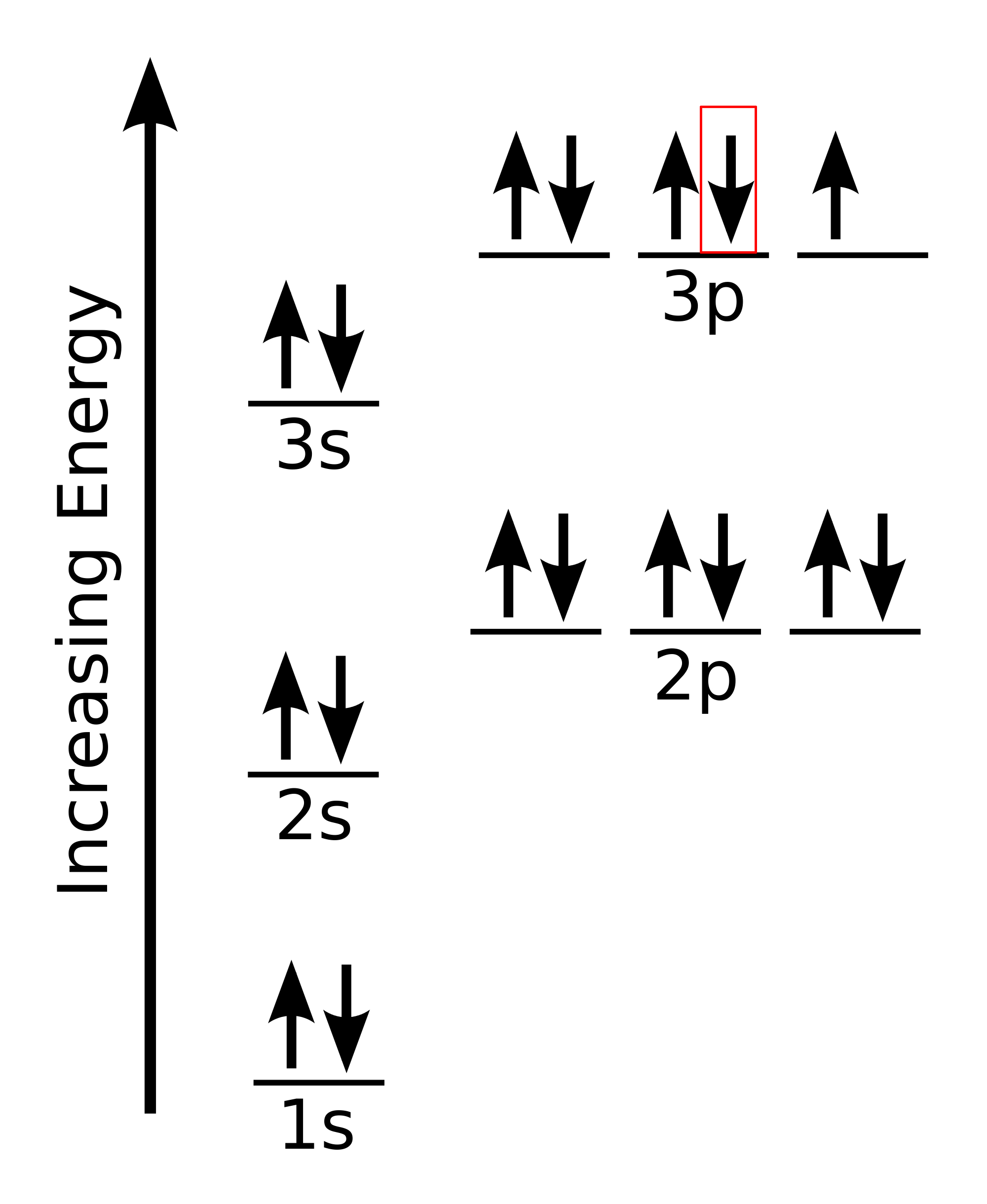
That happens because the empty #3d# orbitals are actually higher in energy than the empty #4s# orbital, as seen here. However, once the #4s# orbital is filled, it becomes higher in energy than the #3d# orbitals. This means that when titanium loses electrons, it does so from the #4s# orbital first. #"Ti: " 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# Ionization energy of atoms, denoted E i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound atomic electrons.The measurement is performed in the gas phase on single atoms. While only noble gases occur as monatomic gases, other gases can be split into single atoms.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...

Orbital diagram of titanium
Orbital Diagram For Aluminum.They consist of the symbol for the element in the. It explains how to write the orbital diagram. Electron Dot Diagram For Aluminum — UNTPIKAPPS (Millie Bailey) Here are some orbital diagrams of elements with more electrons to help you understand the rules, electron configuration, orbital diagrams, and quantum numbers. . They consist of the symbol for the element in 1.11.2021 · Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is known as Ionic charge. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. We thoroughly check each answer to a question to provide you with the most correct answers. Found a mistake? Let us know about it through the REPORT button at the bottom of the page. n atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other … Electron Configuration Practice Quiz Read More »
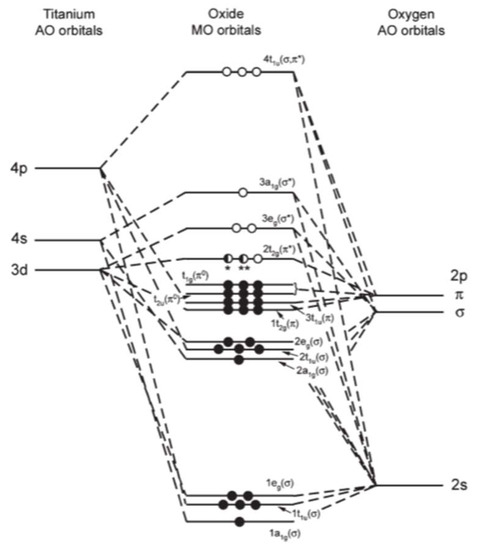
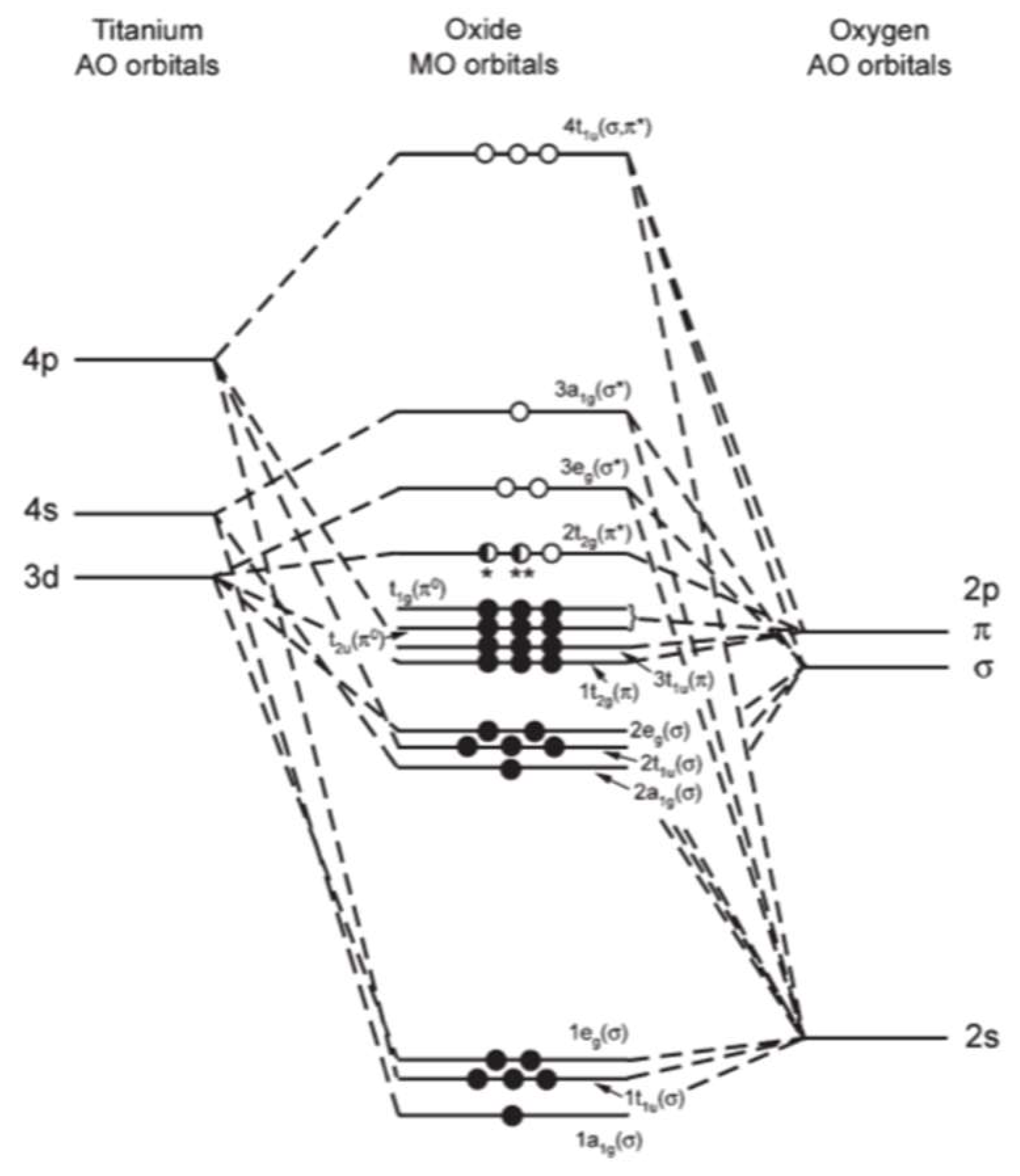
Orbital diagram of titanium. Construct the qualitative molecular orbital diagram for the dinuclear titanium complex from the frontier orbitals of the bent Cp 2 Ti fragment (in C 2v symmetry) and the appropriate frontier molecular orbitals of nitrogen. Label the MO with appropriate symmetry labels, identify the nature of the bond (i.e., σ, σ*, π, π*) and fill up the MO ... Titanium Electron Configuration (Ti) with Orbital Diagram. Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high strength. It is resistant to corrosion in aqua regia, sea water, and chlorine. This element was named by Klaproth. It was nearly a hundred years later (1887) when impure titanium was first prepared by Nilson and Pettersson. About 20 years later Hunter heated Titanium Chloride TiCl 4 with sodium in a steel bomb and isolated 99.6% pure titanium. It is the ninth most abundant element in the earth's crust and is also found in ... Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the data : Calculate the relative atomic mass of titanium to two decimal places. Ans ... Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided.
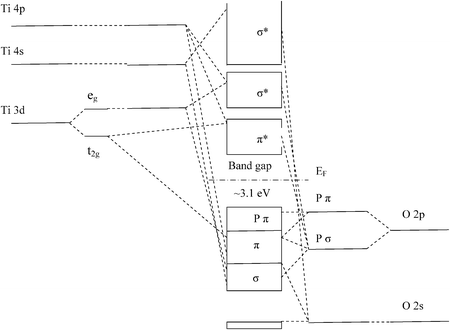
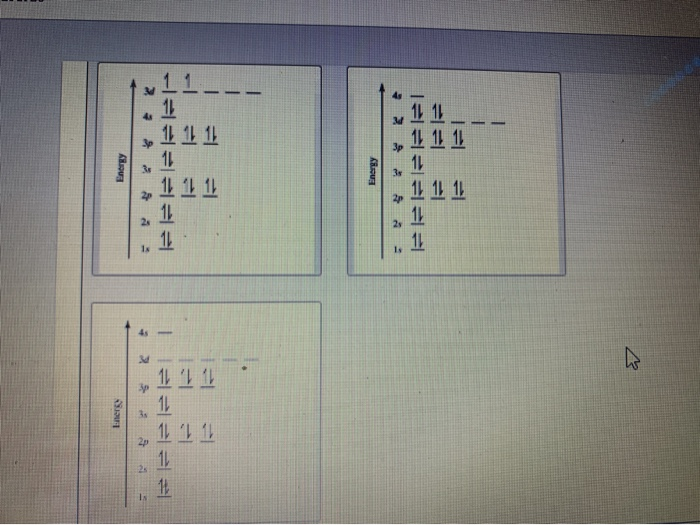
Interaction Diagram. The molecular orbitals of the two extreme geometries 2 and . 4 . are constructed in Figure . 3. In the middle of the figure, there are four d-block orbitals of each Ti fragment, r = 0.2 . A . and . r = 0.0 . A. The . yz . orbital is high above in energy due . to . the strong ligand field of (O,,),, and is not shown. For . r ... For that, we have electron shell diagrams. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom diagram, the element symbol is listed in the nucleus. This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons. Let's consider titanium (Z = 22). Its electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2, which the (n + l) rule correctly predicts. If the electron configuration depended solely on the orbital energies, we would expect: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 - with no electrons in the 4s orbital. Orbital Diagram For Titanium. First you'd follow the filling of orbitals in accordance with the Aufbau principle. This video shows how to draw the orbital diagram of Titanium (Ti). Orbital Diagram For Ti2 — UNTPIKAPPS (Nicholas Hudson) Fill in the electron configurations for the elements given in the table.
Electron binding energies for titanium. All values of electron binding energies are given in eV. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules; relative to the Fermi level for metals; and relative to the top of the valence band for semiconductors. Label Orbital eV ... highest occupied molecular orbital/lowest unoccupied mo-lecular orbital ~HOMO-LUMO! gap. The n1 vibrational fre-quency of the TiO2 ground state is measured to be 960 ~40! cm21. The EA of TiO 2 ~1.59 eV! is only slightly higher than that of TiO ~1.30 eV! while TiO3 has a very high EA of about 4.2 eV. The larger (TiO2)n clusters are all found to be orbital #4s, so you can see here However, once the #4s # orbital is filled, it becomes higher in energy than the orbital#3d #. This means that when titanium loses electrons, it does so from #4s # orbital first. #Ti: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# Therefore, the two electrons that get lost when Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals.
edit: diagram is still missing the sorters to split to the vertical belts for Titanium. 1/s Frame Material is essentially equivalent to 1/6s Rocket, for quick calculations. However, if Stalagmites are available, there's a very specific conditioning that allows for a relatively compact in-line build for Frame Materials with the Nanotubes.
41 orbital diagram for titanium. The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells.
Orbital notation for argon
Ti: 1s22s22p63s23p64s25d2 . It has a total of 22 electrons occupying these orbitals of these quantum numbers.
Astronauts Neil A. Armstrong, Michael Collins and Edwin E. "Buzz" Aldrin Jr., inside the command module of the Apollo 11 Saturn V launch vehicle, rose from Pad 39A at Kennedy Space Center, Florida. The instant of lift-off was 9:32 a.m. EDT, July 16, …
The diagram shows the state of this term with M L = 1 and M S = 1. Rule 2. This rule deals with reducing the repulsion between electrons. It can be understood from the classical picture that if all electrons are orbiting in the same direction (higher orbital angular momentum) they meet less often than if some of them orbit in opposite directions.
Orbital Diagram. 1s ... Titanium dioxide (TiO2), a white pigment that covers surfaces very well, is used in paint, rubber, paper and many others. Sources Usually occurs in the minerals ilmenite (FeTiO3) or rutile (TiO2). Also in Titaniferous magnetite, titanite (CaTiSiO5), and iron ores. Pure metal produced by heating TiO2 with C and Cl2 to ...
This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
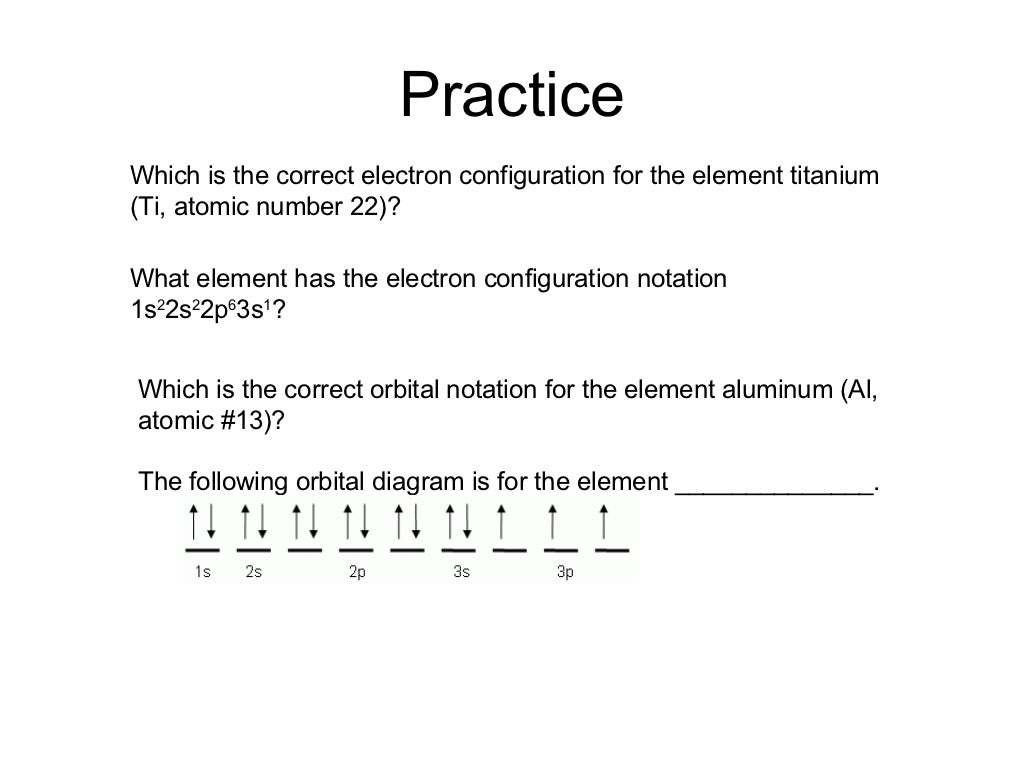
-Titanium •1s 22s22p63s 3p64s23d2. Practice •Potassium . Practice •Potassium -Atomic Number = 19 -1s 22s 2p63s23p64s1 -Superscripts add up to atomic number . The s suborbital ... -Completing orbital diagrams using arrows to represent electrons . Title: Electron Configuration
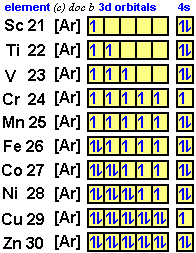
Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium ...
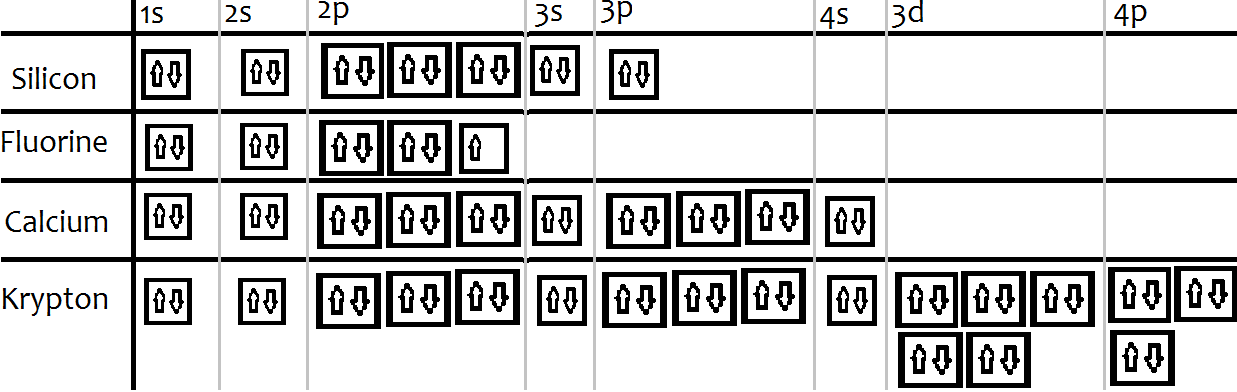
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Titanium is the ninth most abundant element on Earth. It is almost always present in igneous rocks and the sediments derived from them. It occurs in the minerals ilmenite, rutile and sphene and is present in titanates and many iron ores. Titanium is produced commercially by reducing titanium (IV) chloride with magnesium.
Titanium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ...
Orbital Diagram For Chromium Electron Configurations In The 3d Orbitals. Orbital Diagram For Chromium Exam 2013 Chem1010 Fundamentals Of Chemistry Studocu. Orbital Diagram For Chromium Give Electronic Configuration And Orbital Diagram Of Titanium And
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of ...
Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1).
on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so.
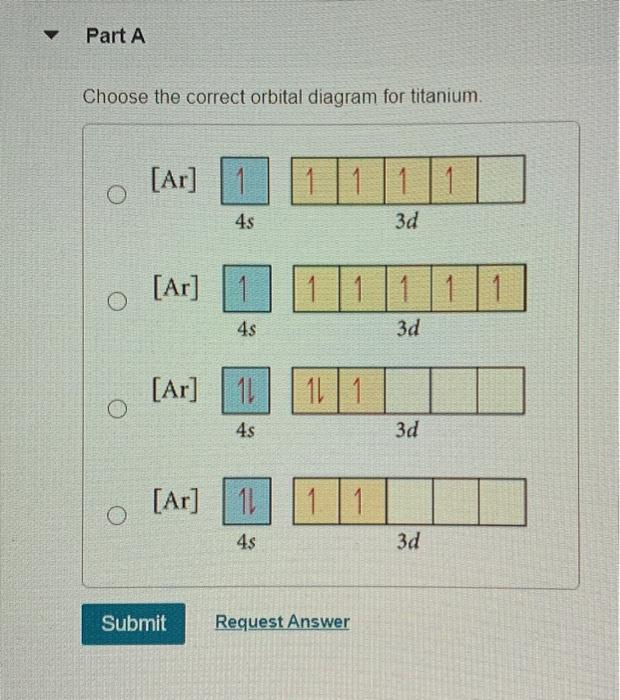
The orbital diagram of titanium is as follows: Become a member and unlock all Study Answers. Try it risk-free for 30 days Try it risk-free Ask a question. Our experts can answer your tough ...
21.10.2021 · The vibrating sea monster of headsets — Razer’s new Kraken gaming headsets bring controller-like vibrations to your head Razer is combining adjustable haptic feedback with its best proprietary ...
We thoroughly check each answer to a question to provide you with the most correct answers. Found a mistake? Let us know about it through the REPORT button at the bottom of the page. n atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other … Electron Configuration Practice Quiz Read More »
1.11.2021 · Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is known as Ionic charge. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed.
Orbital Diagram For Aluminum.They consist of the symbol for the element in the. It explains how to write the orbital diagram. Electron Dot Diagram For Aluminum — UNTPIKAPPS (Millie Bailey) Here are some orbital diagrams of elements with more electrons to help you understand the rules, electron configuration, orbital diagrams, and quantum numbers. . They consist of the symbol for the element in







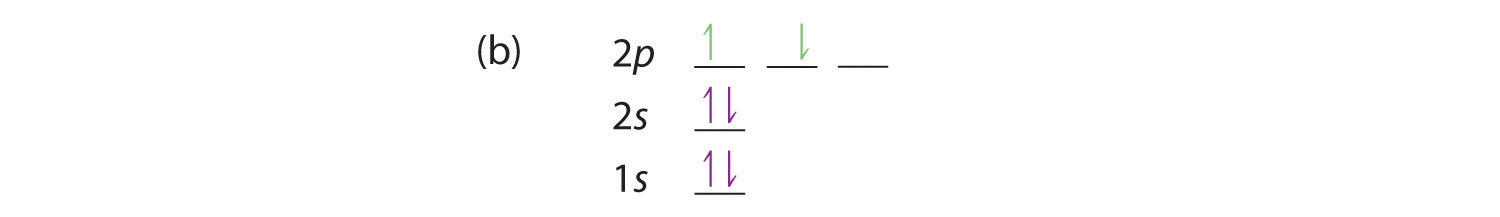









0 Response to "37 orbital diagram of titanium"
Post a Comment