38 study the following phase diagram of substance x.
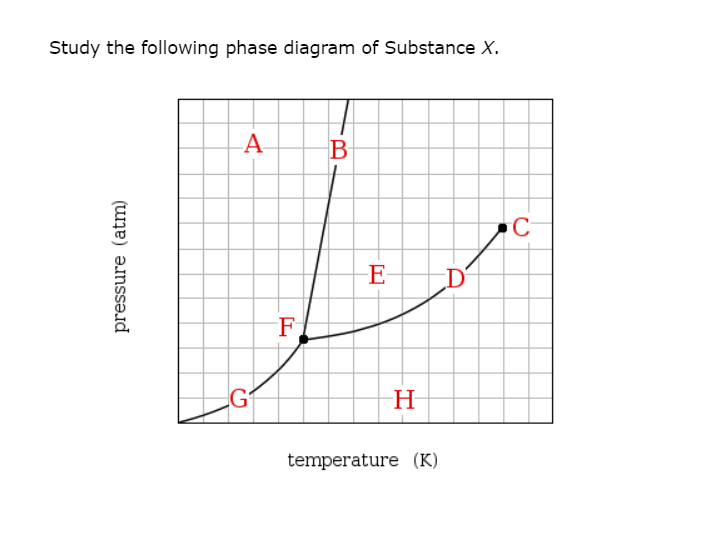
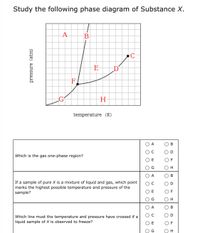
Transcribed image text: Study the following phase diagram of Substance X. pressure (atm) E D temperature (K) Which region includes the lowest pressures and ...
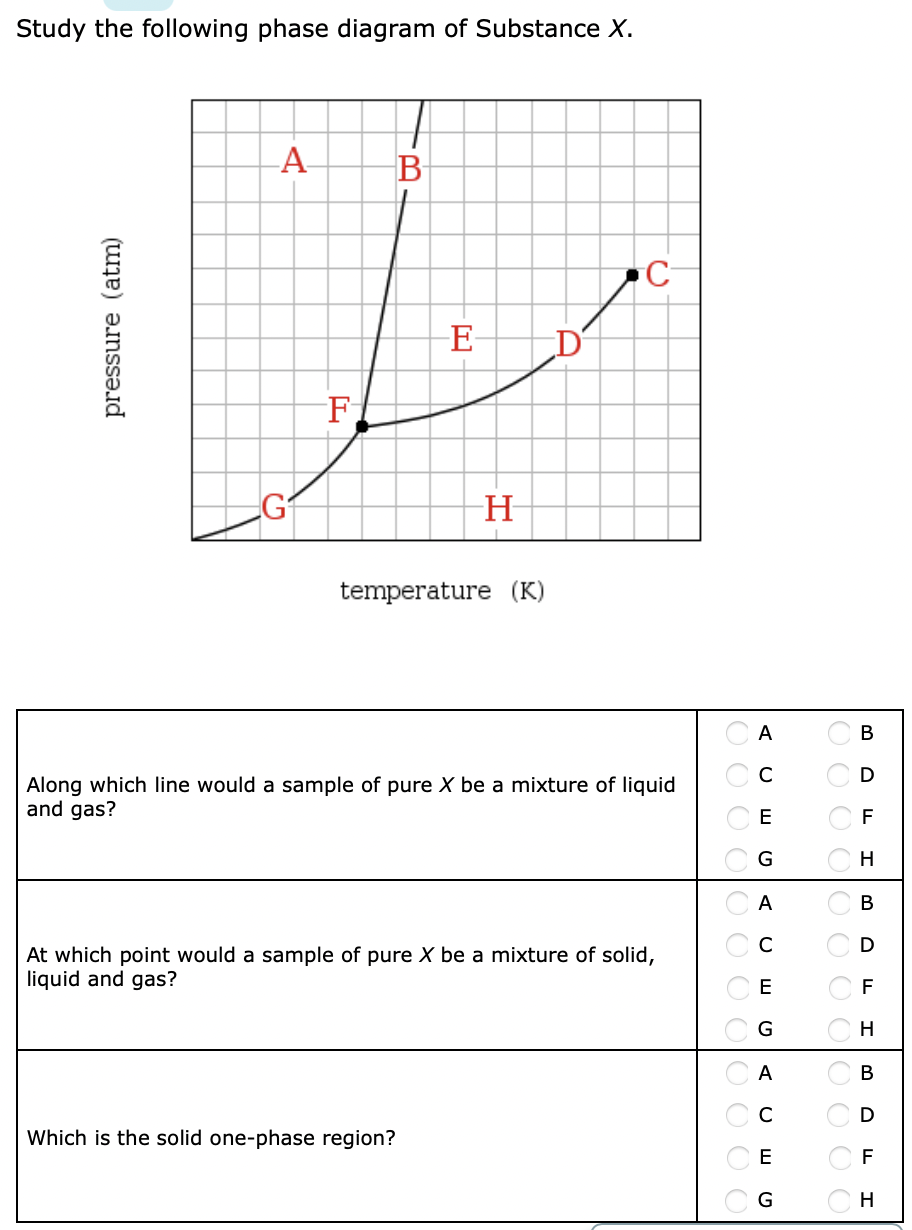
Based on the phase diagram shown above, which of the following statements are correct? I. Sublimation occurs at a point in the transformation that occurs along a straight line from point A to point F. II. C and E represent points where the gas and liquid phases are in equilibrium. III. ∆Hvap can be measured at point B. IV.
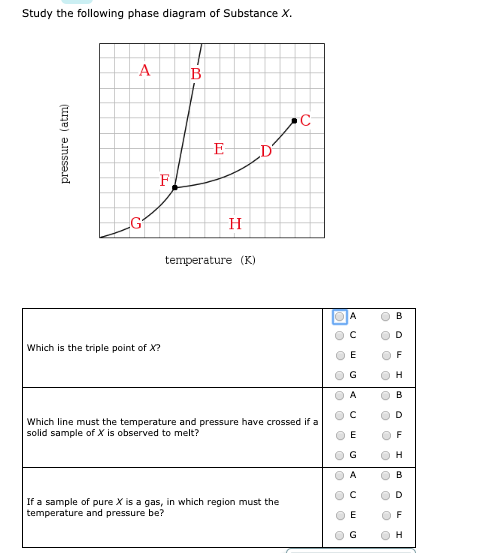
Chemistry. Chemistry questions and answers. Study the following phase diagram of Substance X. pressure (atm) ED temperature (K) Ο Which is the triple point of X? η Ι Φ Ο Which line must the temperature and pressure have crossed if a solid sample of X is observed to melt? OOOOOOOOOOOO η OoooOOOOOO Ι Φ Ο If a sample of pure X is a gas ...

Study the following phase diagram of substance x.
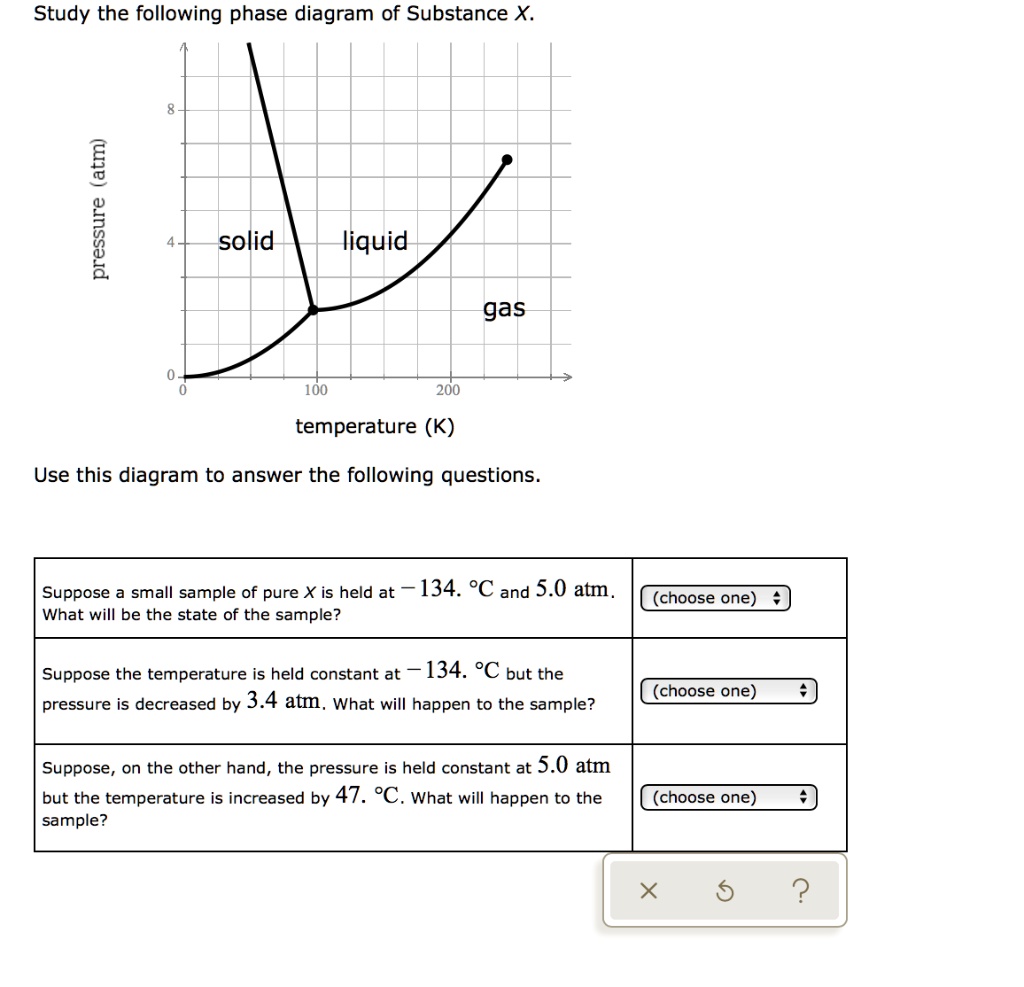
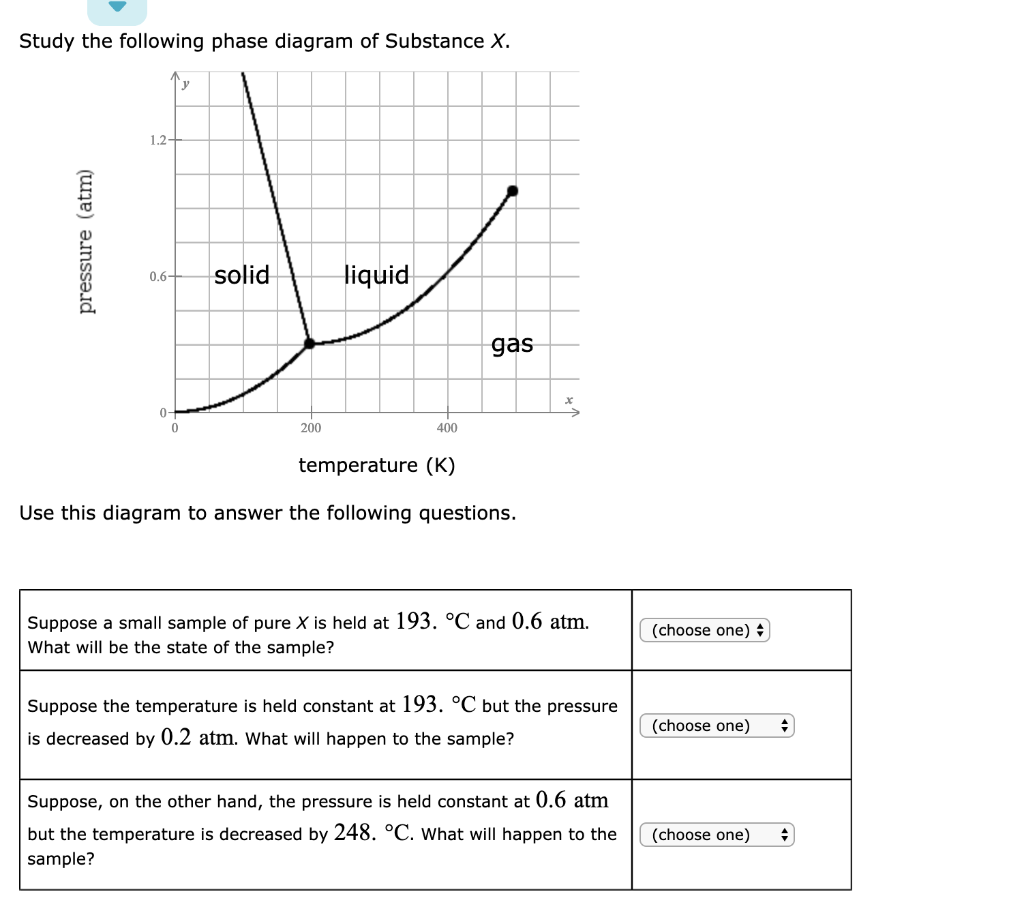
Transcribed image text: Study the following phase diagram of Substance X. 3.2 pressure (atm) 1.6 solid liquid gas 100 200 300 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at - 111. °C and 1.8 atm. What will be the state of the sample? (choose one) e Suppose the temperature is held constant at - 111. °C but the pressure is ...
The simplest phase diagrams are pressure–temperature diagrams of a single simple substance, such as water. The axes correspond to the pressure and temperature. The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of ...
Answer to Use the phase diagram of substance X below to find the melting point X when the pressure above the solid is 1.2 atm. Deg...
Study the following phase diagram of substance x..
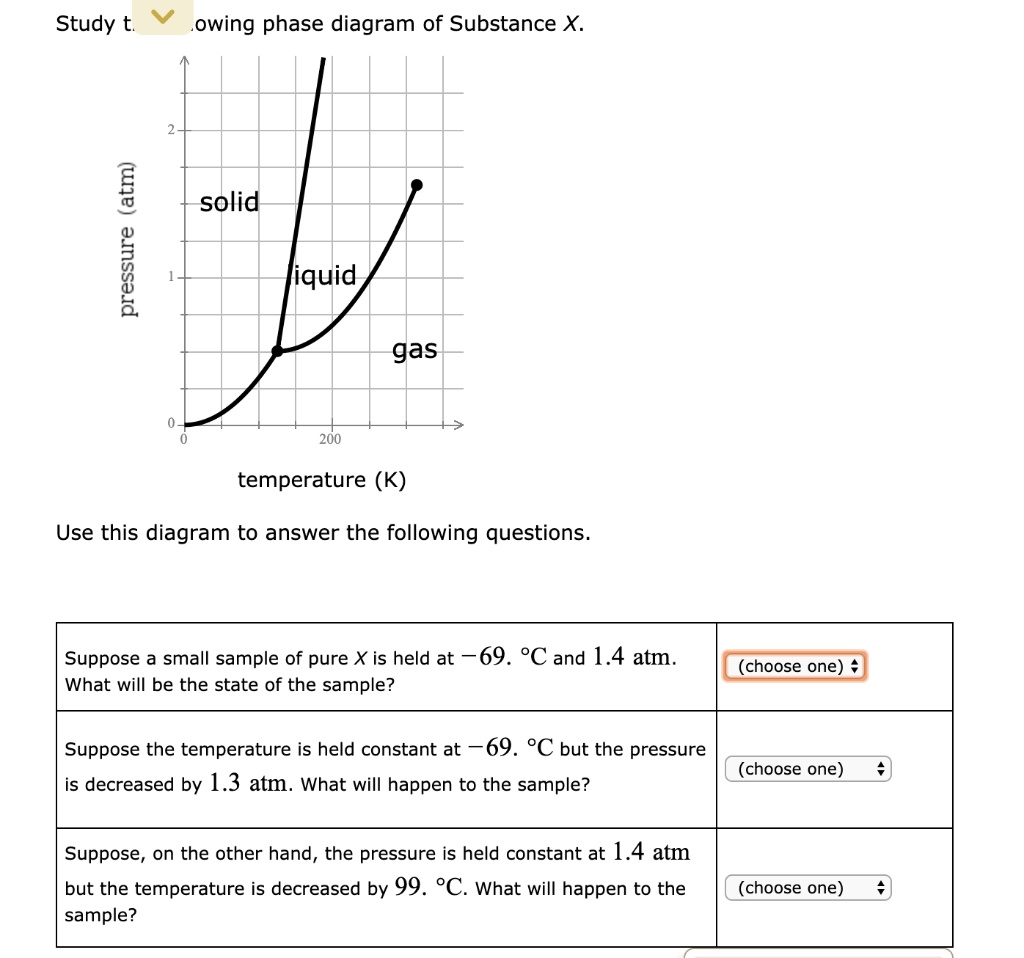
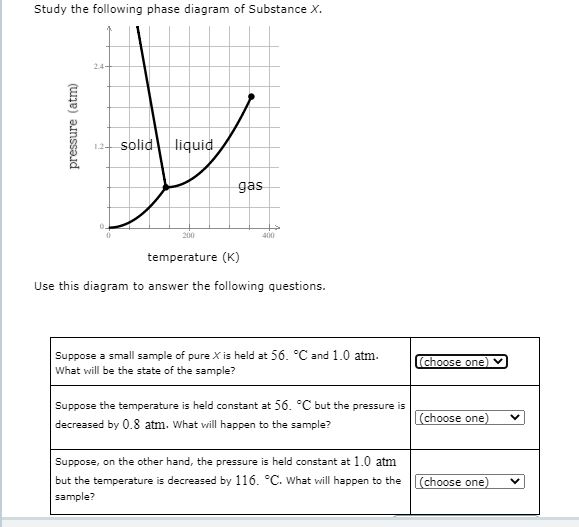
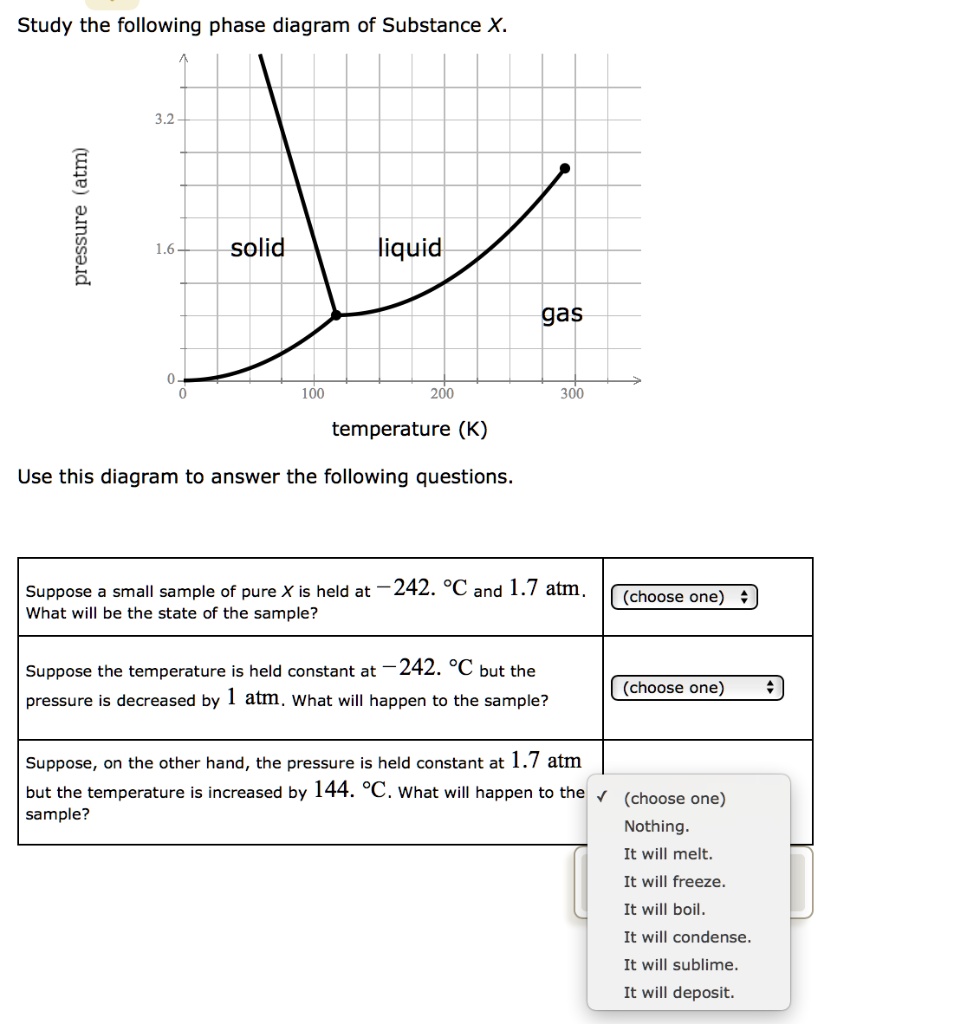
Chemistry Q&A Library Study the following phase diagram of Substance X. 3.6- 1.8- solid liquid gas 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -61. °C and 2.1 atm, What will be the state of the sample? (choose one) Suppose the temperature is held constant at -61. °C but the pressure (choose one) is decreased by 1.5 atm.
Transcribed image text: Study the following phase diagram of Substance X. -A E D temperature (K) Which region includes the lowest pressures and highest ...
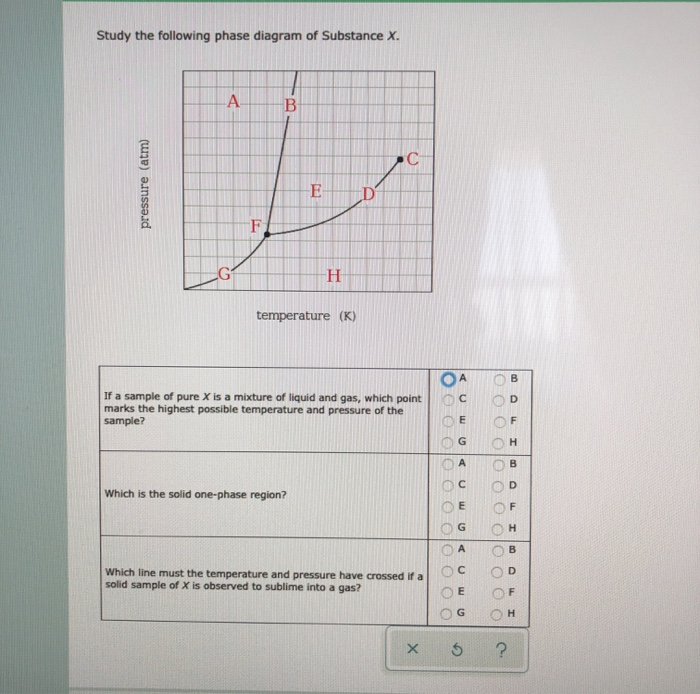
Transcribed image text: Study the following phase diagram of Substance X. AB pressure (atm) ED temperature (K) If a sample of pure X is observed to be a ...
Transcribed image text: Study the following phase diagram of Substance X. 2.4 solid 121 liquid gas 200 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 109. °C and 1.5 atm. What will be the state of the sample? liquid Suppose the temperature is held constant at 109. °C but the pressure is decreased by 1.1 atm.
An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water.
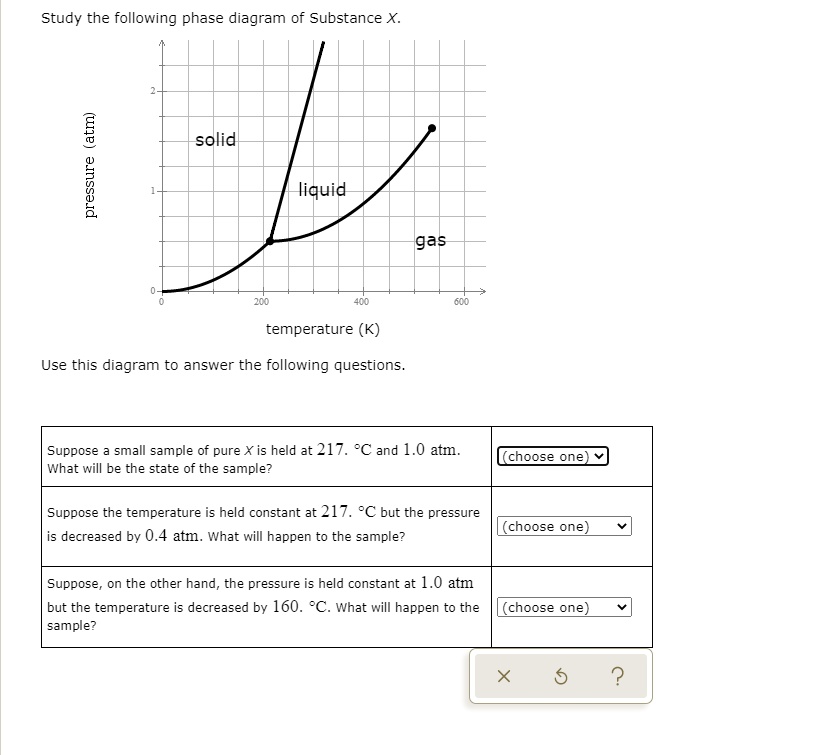
Study the following phase diagram of Substance X. 0.8 pressure (atm) 0.4- solid liquid gas 200 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 2. °C and 0.4 atm.
Study the following phase diagram of substance x. In the phase diagram for substance x what is the triple point of substance x. A phase diagram is a graph of the physical state of a substance solid liquid or gas and the temperature and pressure of the substance. Phase diagrams in the chemistry. Normal boiling point c.
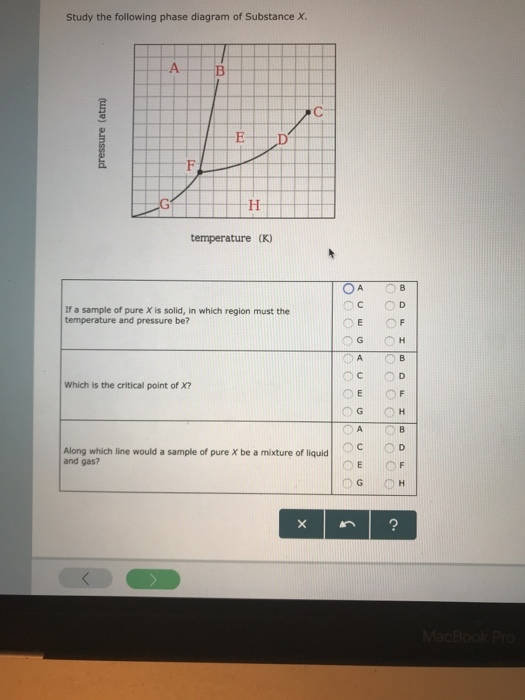
Transcribed image text: Study the following phase diagram of Substance X. pressure (atm) temperature (K) Which is the solid-liquid coexistence curve?
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
1.14. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures? In the phase diagram for substance X, what is the triple point of substance X? Nice work! You just studied 5 terms!
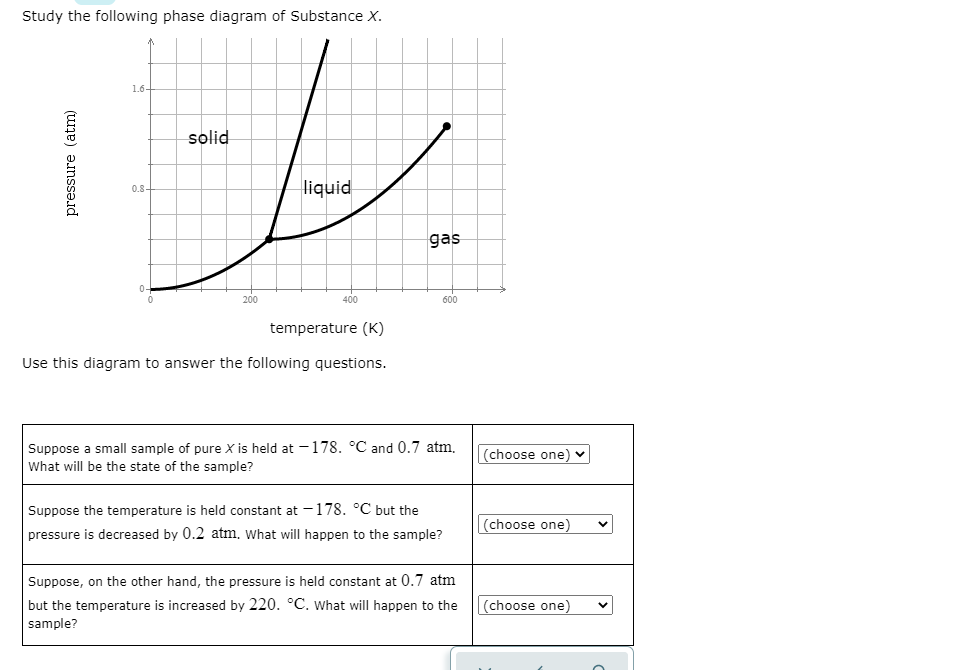
Study the following phase diagram of Substance X. 1.6+ solid 0.8- liquid gas 200 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -178. °C and 0.7 atm.
Transcribed image text: Study the following phase diagram of Substance X. pressure (atm) temperature (K) Which line must the temperature and pressure have crossed if a liquid sample of X is observed to freeze? If a sample of pure X is to be a mixture of liquid and gas, which point marks the lowest possible temperature and pressure of the sample?
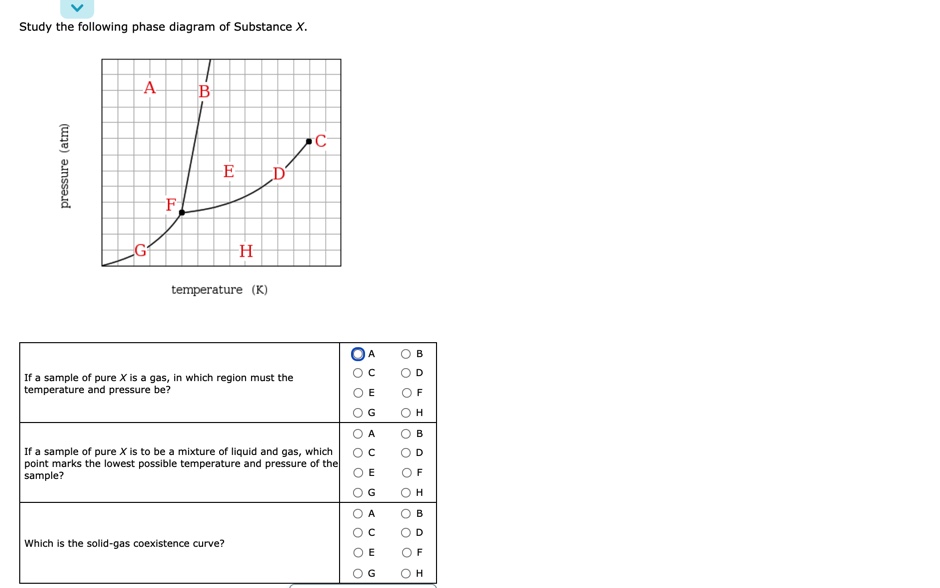
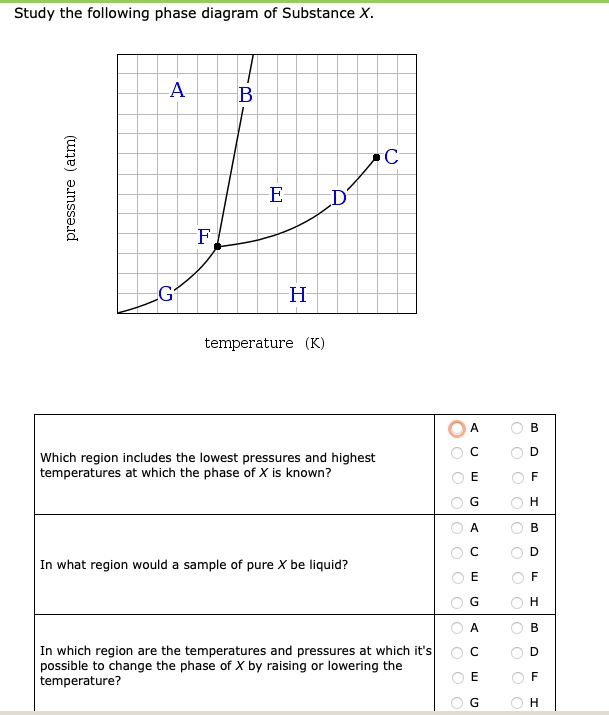
Study the following phase diagram of Substance X. A А B С pressure (atm) E D F G H temperature (K) In what region would a sample of pure X be a gas? OA OC OE OG ОА Ос OE OG OB OD OF OH OB OD OF OH OB OD If a sample of pure X is a mixture of liquid and gas, which point marks the highest possible temperature and pressure of the sample?
Unlike most other substances, the density of solid H2O is than the density of the liquid; hence water when it freezes. This is reflected in the phase diagram for H2O (shown). The solid-liquid boundary line slopes upward to the , reflecting the fact that the higher the pressure the the temperature at which the water freezes.
Phase diagram: The diagram which represents the three states of a substance in single space with respect to the temperatures and pressures involved in all its three phases is said as the phase ...
Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist ...
Chemistry Unit 1 Lesson 14. 1. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures? 2. In the phase diagram for substance X, what is the triple point of substance X? Refer to the phase diagram for substance X in Problem Set 60: Phase Diagrams, in the Chemistry: Problems and Solutions book.
Study the following phase diagram of Substance X. solid liquid gas pressure (atm) check_circle.
Answer to Study the following phase diagram of substance X. temperature (K)...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Temperature k study the following phase diagram of substance x. Phase diagrams wiva k12 chemistry. This is the phase diagram for a typical pure substance. Which point marks the highest temperature and pressure at which its possible to determine whether a sample of pure x is a liquid or a gas. Phase diagram is a graphical representation of the ...
Transcribed image text: Study the following phase diagram of Substance X. pressure (atm) s+ solid liquid gas temperature (K) Use this diagram to answer the ...
18. The phase diagram for the pure substance X is shown on the right. The temperature of a sample of pure solid X is slowly raised from 10°C to 100°C at a constant pressure of 0.5 atm. What is the expected behavior of the substance? (A) It first melts to a liquid and then boils at about 70°C.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Stay up to date with free content alerts via e-mail · All publications more feeds
The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, ...
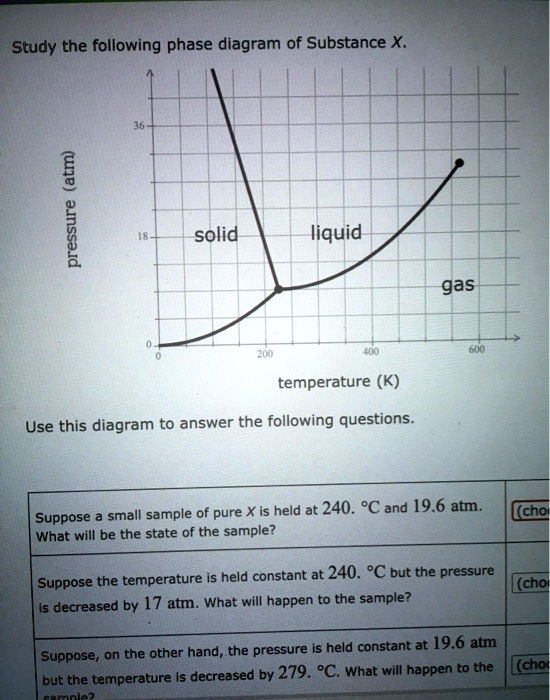
Consider the phase diagram for substance X shown below. Which of the following is NOT True for substance X? a. At the conditions indicated by point C, substance X will be a liquid. b. At the conditions indicated by point B, substance X will be a gas. c.
May 3, 2021 - Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and …
Transcribed image text: Study the following phase diagram of Substance X. pressure (atm) temperature (K) g 0 If a sample of pure X is a mixture of liquid ...
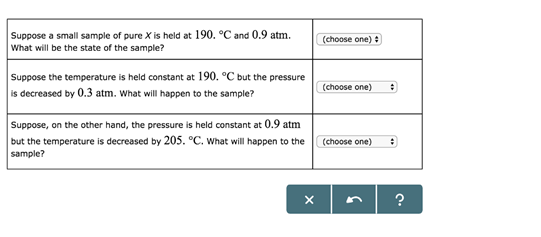
Study the following phase diagram of Substance X. 1.6- pressure (atm) 0.8 solid liquid gas 200 400 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -48. °C and 0.9 atm. What will be the state of the sample? (choose one Suppose the temperature is held constant at - 48. °C but the ...
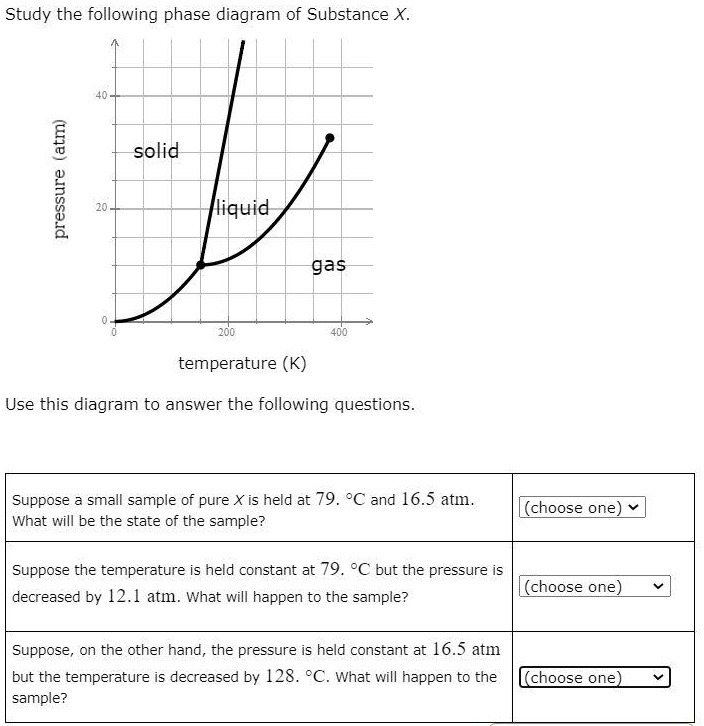
Chemistry Q&A Library Study the following phase diagram of Substance X. 16- solid liquid gas 100 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at What will be the state of the sample? 104. °C and 6.1 atm. (choose one) v (choose one) Suppose the temperature is held constant at - 104. °C but the pressure is increased by 4.9 atm.
Transcribed image text: Study the following phase diagram of Substance X. solid pressure (atm) liquid gas 300 400 temperature (K) Use this diagram to answer the following questions. (choose one) Suppose a small sample of pure X is held at -221 °C and 1.1 atm What will be the state of the sample? (choose one) Suppose the temperature is held constant at -221. °C but the pressure is decreased ...
Phase Diagrams. Open the phase diagram for CO2 given in the introduction again. Use the phase diagram for CO2 in the interactive activity and determine which of the following statements are correct. CO2 is a gas under normal conditions of temperature and pressure. All three phases of CO2 exist simultaneously at the triple point.
Study the following phase diagram of Substance X. 1.2 solid pressure (atm) 0.0 liquid gas 100 200 300 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at - 121. °C and 0.5 atm What will be the state of the sample?
Study the following phase diagram of Substance X. solid pressure (atm) liquid gas temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -202. °C and 1.2 atm. What will be the state of the sample? (choose one) Suppose the temperature is held constant at - 202. °C but the pressure is ...
Study the following phase diagram of Substance X. 3.2- solid pressure (atm) 16 liquid gas 100 temperature (K) Use this diagram t 43. °C and 2.4 atm. choose one) e Suppose the temperature is held constant at 43. °C but the pressure is decreased by 2 atm.
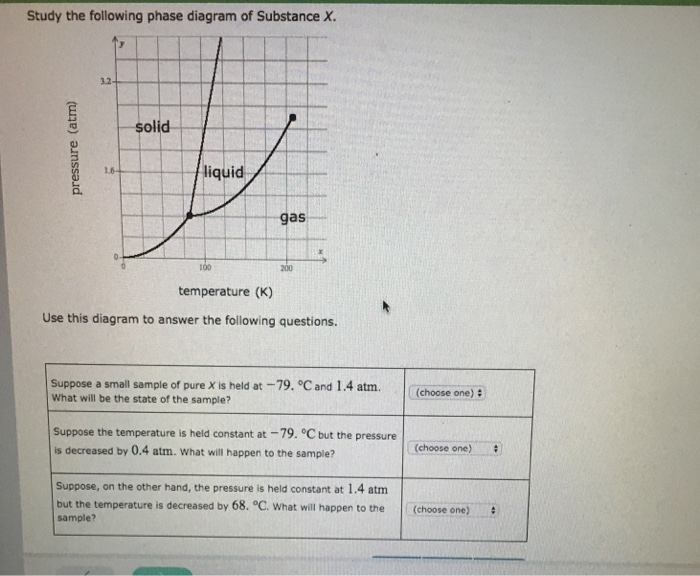
Transcribed image text: Study the following phase diagram of Substance X. solid liquid gas 100 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at-79. °C and 1.4 atm. What will be the state of the sample? (choose one) Suppose the temperature is held constant at-79. °C but the pressure is decreased by 0.4 atm.
A typical phase diagram for a pure substance is shown in . The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in . The pressure and temperature axes on this phase diagram of water are not ...
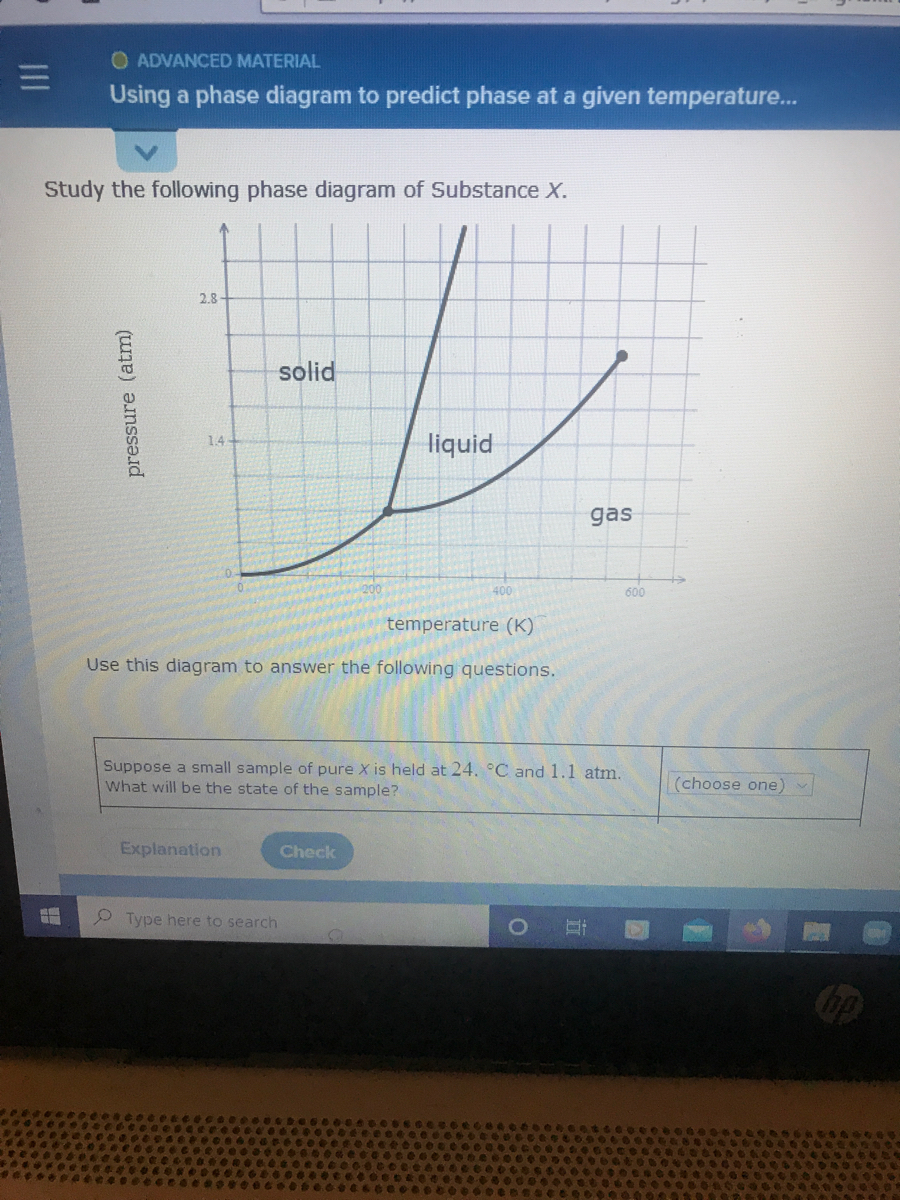
Solution for Study the following phase diagram of Substance X. 2.8 solid liquid 14 gas 400 600 temperature (K) Use this diagram to answer the following…
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation













0 Response to "38 study the following phase diagram of substance x."
Post a Comment