39 orbital diagram for f ion
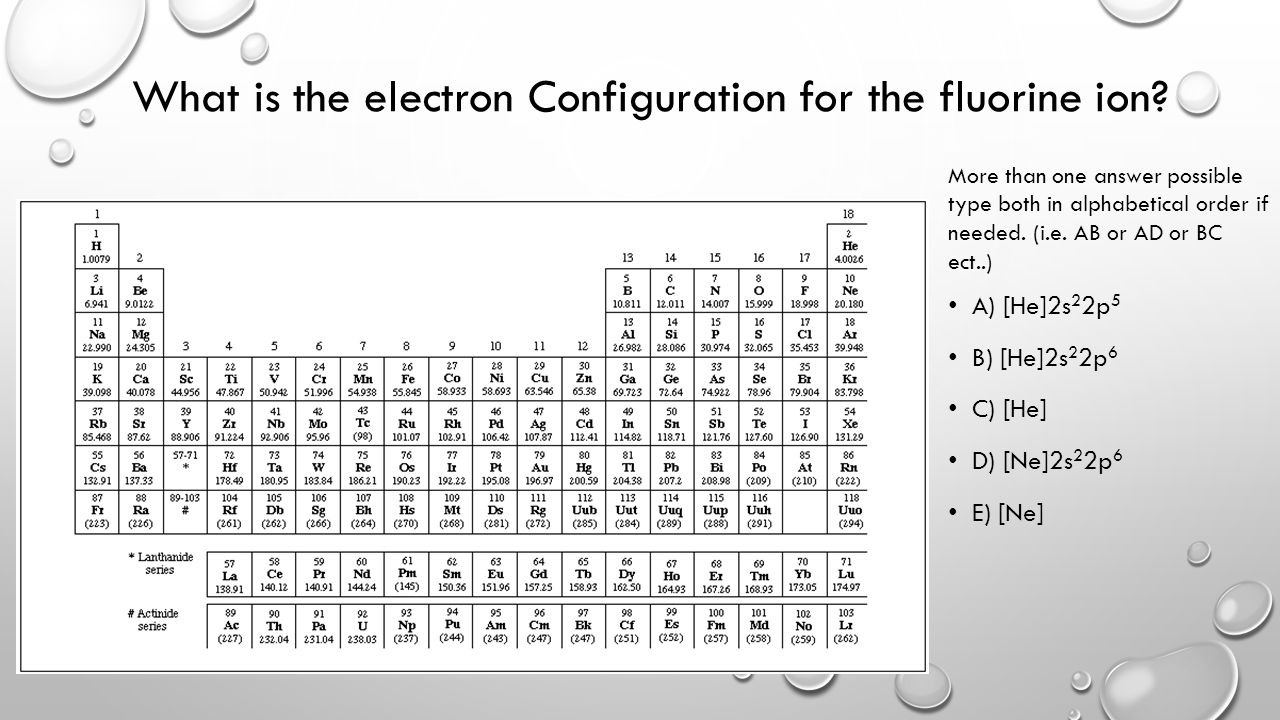
1 answerAnswer: The correct ground state electron configuration of F− − ion is option c. 1s22s22p6. 1 s 2 2 s 2 2 p 6 . Explanation: The atomic...
Hence, three sp2 hybrid orbitals are formed whereas the 2pz orbital remains unchanged and hence, 2pz orbital is used for the formation of a pi bond with the oxygen atom. The sideways overlapping of atomic orbitals results in the formation of a pi bond. The orbital diagram of nitryl fluoride, representing only the sigma bonds, is shown below.
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 subshell? True or False 1.The contour of the orbital would extend further out along the x and y axes. 2. The value of ℓ would increase by . chemistry. Build the orbital diagram for the ion most likely formed by phosphorus. science

Orbital diagram for f ion
Jun 23, 2016 · 1 answerF−:1s22s22p6. Explanation: A good starting point for when you must find the electron configuration of an ion is the electron configuration ...
Subkulit p = 3 orbital maksimal berisi 6 elektron Subkulit d = 5 orbital maksimal berisi 10 elektron Subkulit f = 7 orbital maksimal berisi 14 elektron. Diagram tingkat energi menurut asas aufbau: 1s 2s 2p 3s 3p 4s 3d 4d 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p. Asas larangan Pauli. Setiap orbital diisi maksimum 2 elektron dengan spin yang berlawanan.
Answer to Write orbital diagram for Co2+. Draw The Orbital Diagram For Ion Co 2 Clutch Prep. Sigma Pi Bonding Atomic Orbital Bonding Sigma S 1) Draw the octahedral crystal field splitting diagram for each metal ion. a) V3+ b) Co2+ (high-spin) 2) The [CrCl6]3− ion has a maximum in its absorpt ion spectrum at 735 nm.
Orbital diagram for f ion.
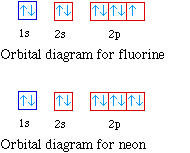
Fluorine (F) has an atomic mass of 9. ... Electron Configuration, [He] 2s2 2p5. 1s2 2s2 2p5. Orbital Diagram ... Lewis Dot Diagram of Fluorine (F).
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbital s (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2 + Molecular Orbital Diagram s of Diatomic Molecules ...
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
Ion electron confugurat ion s. A neutral fluorine atom has 9 electrons. Show transcribed image text construct the orbital diagram of the f ion. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+.
Molecular orbitals are of two types - bonding and antibonding. The two types of bonds are σ - bond and π − bond. The s -orbitals of one atom can overlap with the s, p, d, f, orbital of another atom such that the overlapped region is symmetrical about the internuclear axis. Similar symmetrical overlaps are also possible among p, d and f ...
To accommodate the electrons shared in these bonds, it needs to form 4 hybrid orbitals. As a result, there is a formation of one s-hybrid orbital and three p-hybrid orbitals. ( Each s orbital can accommodate 2 electrons, and p orbital can accommodate 6 electrons). Hence SO42- ion has an sp3 hybridization. SO42- Molecular Geometry
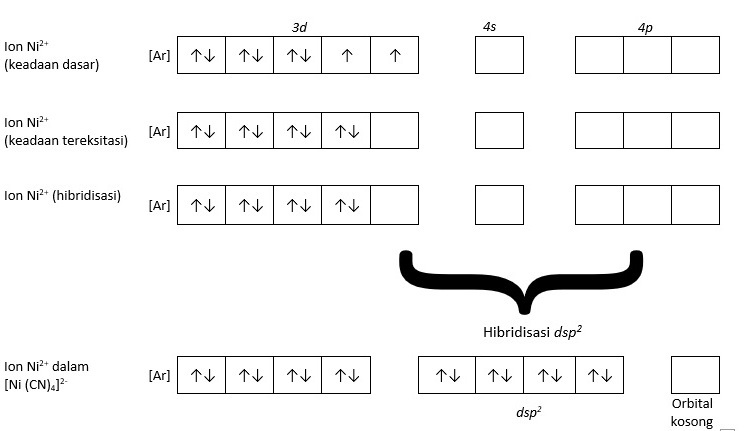
Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the orbitals are represented by half arrows. Explanation: Electronic configuration of Nickel ( 28Ni) is. 1s2 2s2 2p6 3s2 3p6 4s2 3d8.
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Molecular orbital diagram for c2 2- 5 2 10 10 C C 2 2 20 20 C C FIG. 2.
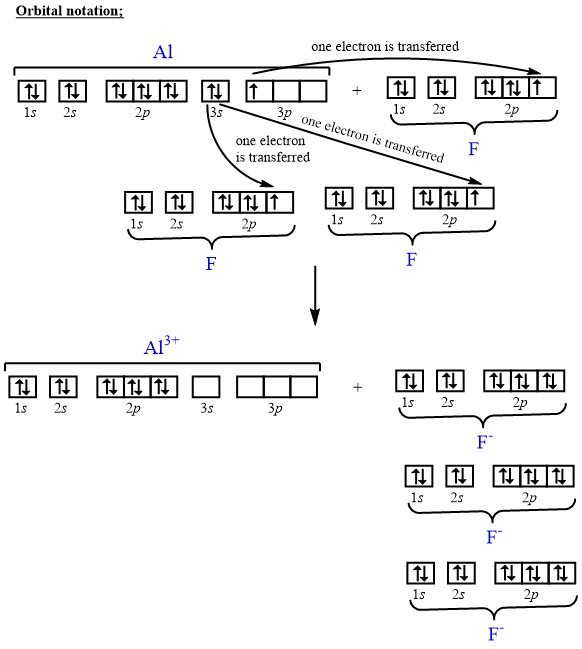
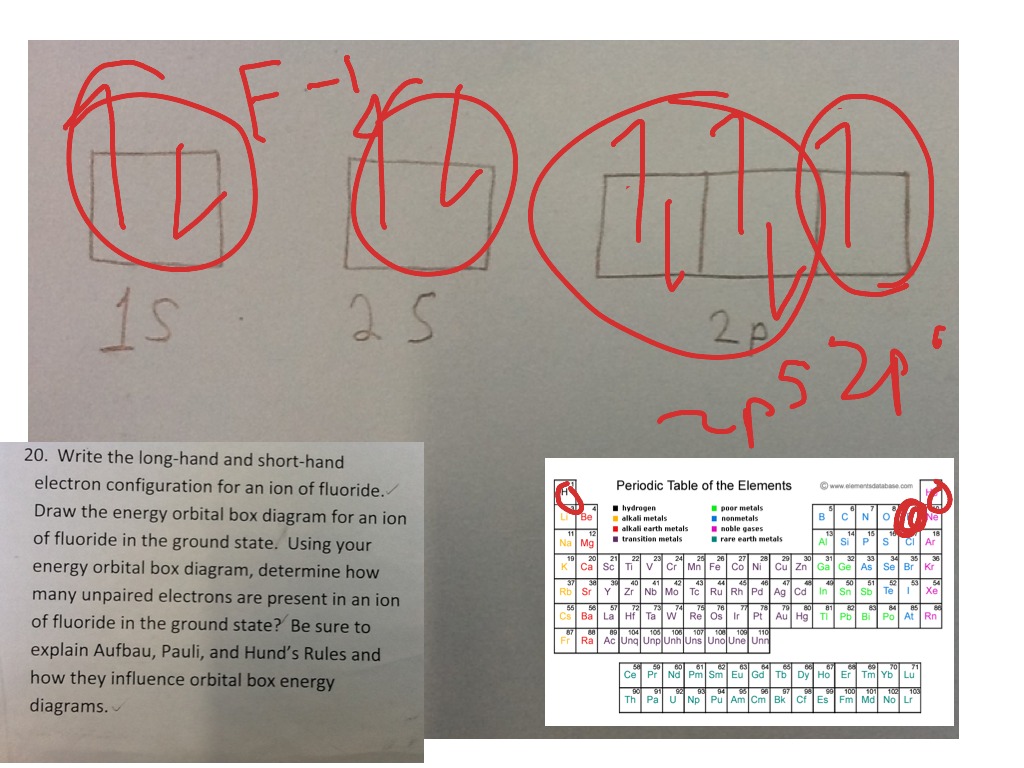
Does the Fluorine (F) atom gain or lose electrons to become an ion? Justify your response using an electron configuration or orbital diagram.
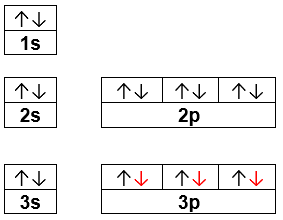
Q. Construct the orbital diagram of the F- ion.A neutral fluorine atom has 9 electrons. How many electrons does a F- ion have? Solved • Apr 30, 2020 The Electron Configuration: Ions Q. What element forms an ion with an electronic configuration of [Ne] and a -2 charge? Give the symbol for the element. ...
Jan 21, 2021 — The electronic configuration for the Fluorine ion is 1s22s22p5 and in this configuration, Fluorine needs 1 electron so as to complete the 2p ...
Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is known as Ionic charge. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed.
Biasanya orbital p digambarkan menggunakan satu kumpulan sumbu x, y, dan z, sehingga diberi tanda px, py dan pz. Orbital d dan f. Setiap subkulit d terdiri atas 5 orbital dengan bentuk kelima orbital yang tidak sama. Orientasi orbital d dilambangkan dengan dxy, dxz, dyz, dx2-y2 dan dz2.
Transcribed image text: Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. How many electrons does a F^- ion have?
Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen. ... When we talk about the orbital ...
Orbital diagram of f ion. The remaining five electrons will go in the 2p orbital. The following is the diagram for the neutral oxygen. Electron configurat ion s orbital diagram s. An orbital diagram naturally leads to the writing of an electron configurat ion. Ion electron confugurat ion s. Construct the orbital diagram of the F^- ion.
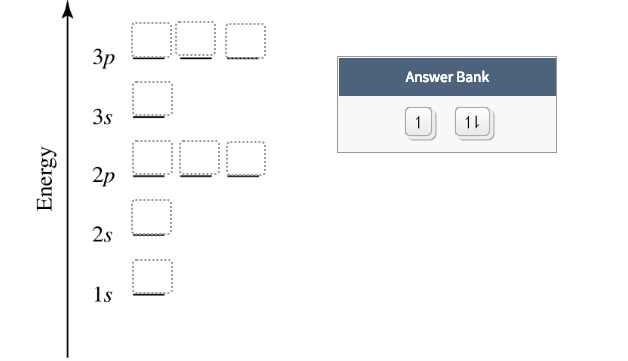
The sublevel 2p has 3 orbital s (2p x, 2p y, and 2p z) and each of the se orbital s has its own line (or box). Let's begin th is sect ion with the orbital box (or the orbital representat ion diagram) for a neutral atom. Draw the electronic configurat ion for potassium using the electron configurat ion diagram below. Remember that potassium is ...
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
Electron configurations orbital notation key electron. If your periodic table doesnt agree with this your answers for elements near the f. The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. The 6 key answers for the electron configuration chem worksheet 5 are.
The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule.
1 answerA neutral fluorine atom has 9 electrons. How many electrons does a F- ion have? FREE Expert Solution. We are asked to construct the orbital diagram of ...
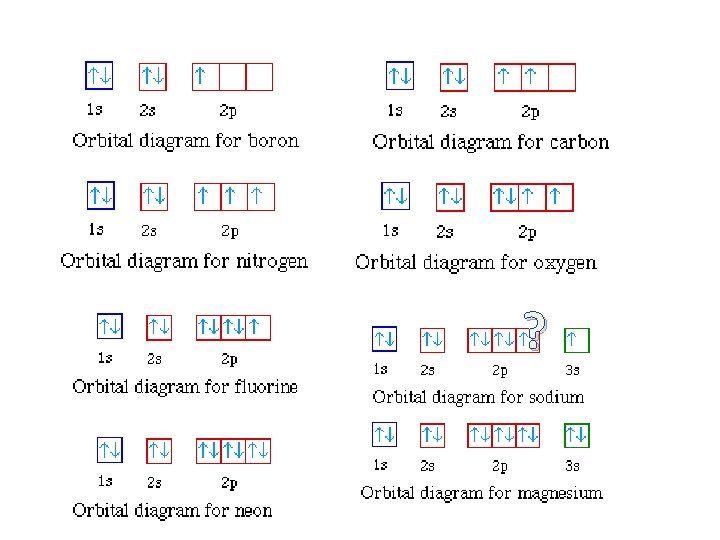
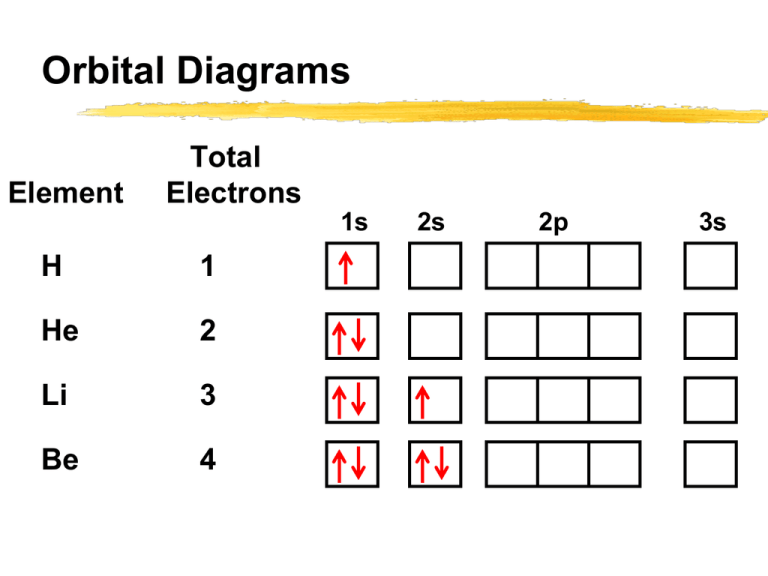
Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ...
Answer (1 of 3): In O2 2+, there is 14 electrons. So, it's MOT is comparable to N[code ]2[/code] & the MOT diagram will look like this :
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
Build the orbital diagram for the ion most likely formed by phosphorus. Does anyone know what it is and how to answer it on mastering chemistry, that **** is so confusing. 1s2 2s2 2p6 3s2 3p6. build the orbital diagram for the ion most li. Orbital Diagram For Phosphorus. This Site Might Help You. RE:
In the molecular orbital diagram for O 2 + ion, the highest occupied orbital is (a) σ MO orbital (b) π MO orbital (c) π* MO orbital (d) σ* MO orbital. Answer. C. Question. The theory capable of explaining paramagnetic behaviour of oxygen is (a) resonance theory (b) V.S.E.P.R. theory (c) molecular orbital theory
According to our diagram, there are 8 bonding electrons and 6 antibonding electrons, providing a bond order that (8 − 6) ÷ 2 = 1. Thus F2 is guess to have a secure F-F single bond, in commitment with experimental data. Example (PageIndex3): Diatomic Sulfur. Use a qualitative molecular orbital energy-level diagram to predict the electron ...
Ionic properties of fluorine atoms — Ionic properties of fluorine atoms. Fluorine is an anion element. When a charge-neutral atom receives an ...
Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, σ 2 p z ⋆ are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1.











![Electron Configuration | Chemistry [Master]](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/1941/2017/05/30162457/cg10c3-010.png)











0 Response to "39 orbital diagram for f ion"
Post a Comment