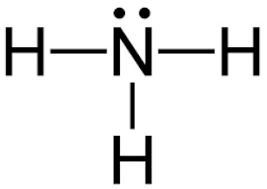
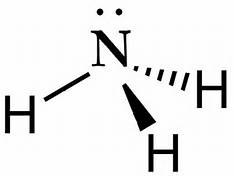
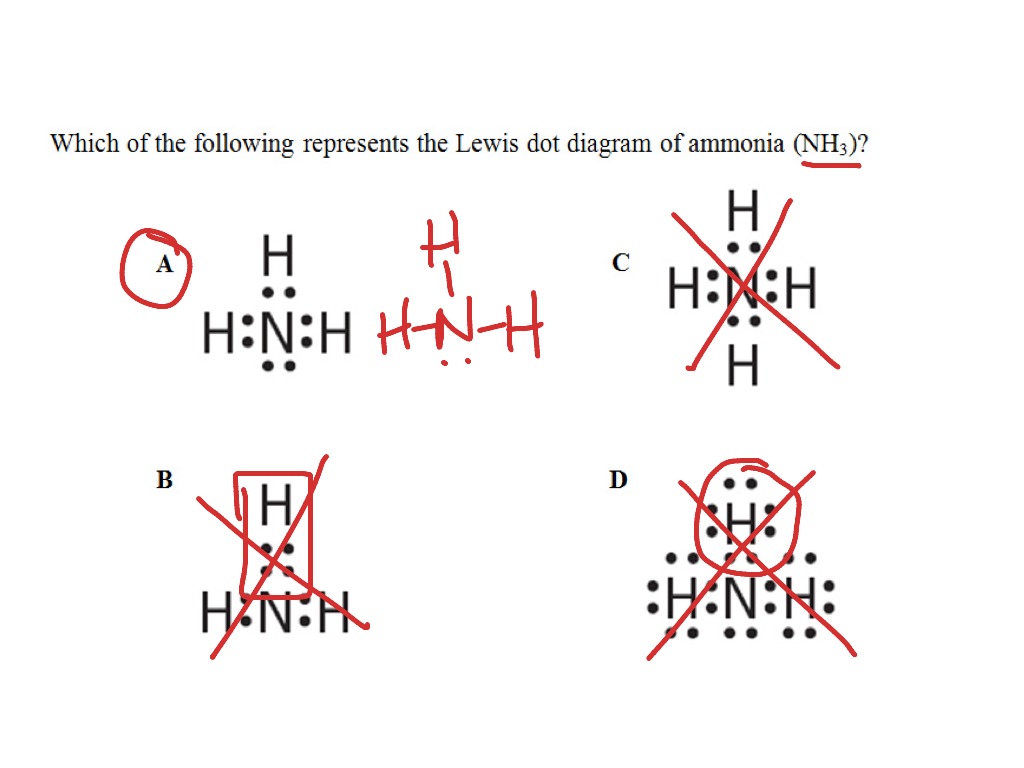
39 which of the following represents the lewis dot diagram of ammonia (nh3)?
Here is the Lewis structure of CO 2. CO2 Lewis structure. By following the above steps, you can draw the Lewis structure of any molecule. Here are some examples. Try to apply these five steps to the following examples and check whether your success or not. CO Lewis structure Step 01: calculation of total valence electrons of CO Step 02:
25 Feb 2019 — Lewis dot diagram nh3. We show two ways to draw the nh3 lewis structure ammonia. It has one valence electron but we have 3 hydrogens so lets ...
How many dots belong in the electron dot diagram of a boron (b) atom? 29/11/2021 · Boron is a unique element in terms of electron deficiency and Lewis acidity. In corporation of boron atoms in to an arom. carbon framework of fers a wide variety of functionality.
Which of the following represents the lewis dot diagram of ammonia (nh3)?
Some will be able to: • Use dot-and-cross diagrams to represent covalent bonding in molecular ions. 12/01/2021 Keywords: Covalent bond Lone pair Displayed formula Dative covalent or Coordinate bond 12/01/2021 Key definitions: A Covalent bond is the strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded ...
Bonding Energy Lewis Structures and VSEPR Worksheet 1. 2 For each of the following compounds a Lewis structure determine the bond angles and molecular shapes for all atoms. Lewis Structure And Vsepr Models. This information given a dot structure and lewis vsepr worksheet with. H2O 8 4 Tetrahedral Bent CO2 G-NH3 5-3 BF3.
The lewis structure also called as electron dot structure is a simplified method of representing the number of valence electrons present within an atom or a molecule. A step by step explanation of how to write the lewis dot structure for carbon monoxidefor the co lewis structure calculate the total number of valence elec.
Which of the following represents the lewis dot diagram of ammonia (nh3)?.
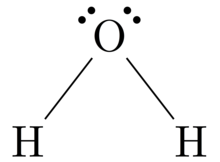
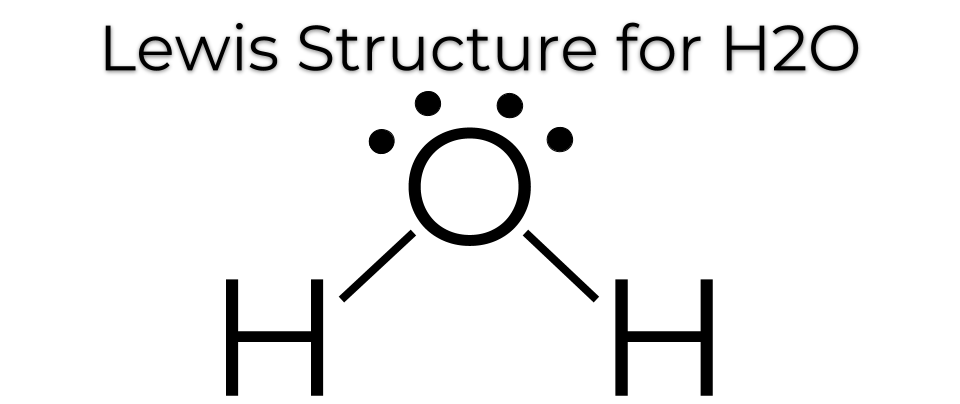
Lewis Structure of Water Each line represents the sharing of 2 electrons. Such compounds are called electron-deficient compounds. A) A Lewis structure in which there are no formal charges is preferred. ShowMe is an open learning community featuring interactive lessons on a variety of topics. Lewis Dot Structure of Atoms Link. Cf4 Lewis Structure.
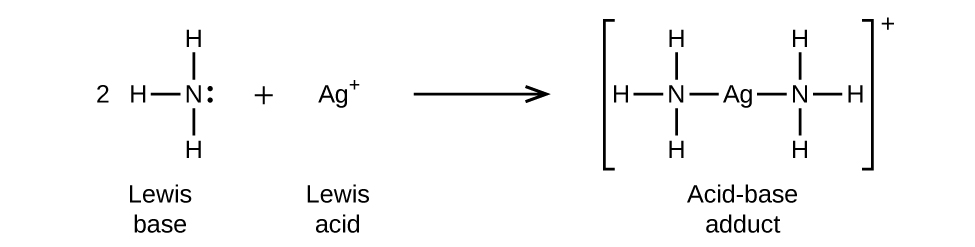
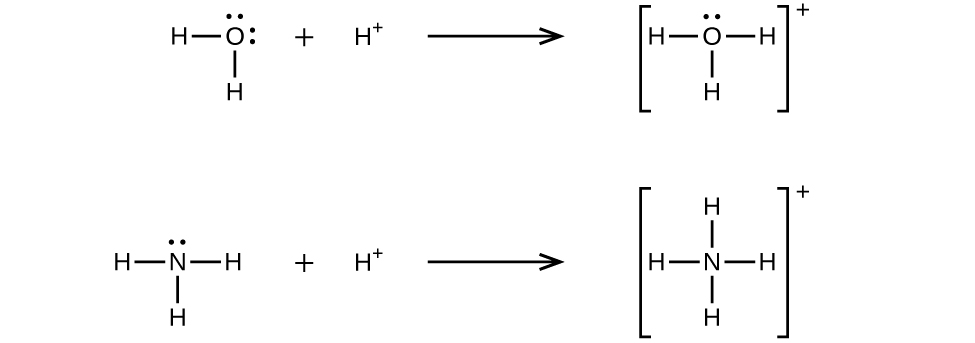
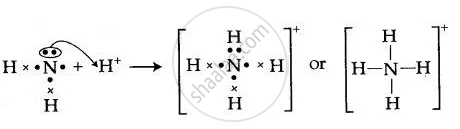
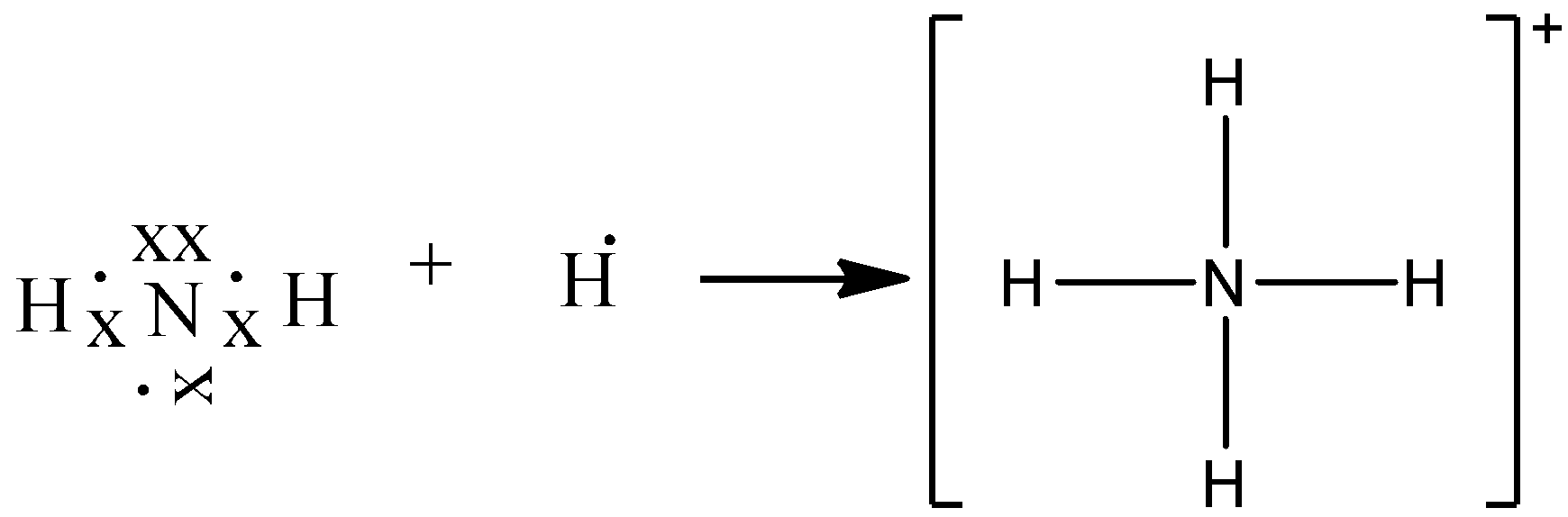
The ion is the by-product of a chemical reaction between a proton donor and Ammonia, which is as follows: NH3 + H+ ——> NH4+ Lewis Structure Lewis Structure is a simplified arrangement and presentation of the electrons present in the valence shell of a molecule.
Lewis Structures for NH3. Step-by-step tutorial for drawing the Lewis Structure for Ammonia.25 Oct 2016 · Uploaded by Wayne Breslyn
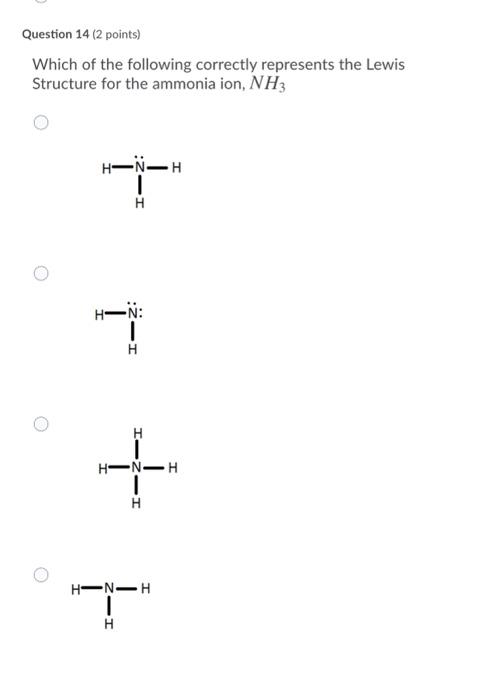
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
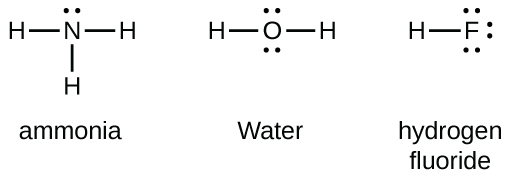
5 May 2018 — The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons ...1 answer · Have a look here... Explanation: The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone ...
What is the correct Lewis Dot Structure for ammonia NH3. ... Q. Which of the following is the correct LD Diagram for Hydrogen Cyanide? HCN? answer choices.
Worksheet 4-04 Arrange the bonds in each of the following sets in increasing polarity. Chemical bonding in metals is a. Learn vocabulary terms and more with flashcards games and other study tools. A chemical bond is an attraction between atoms in order to create compounds made of two or more atoms. Lewis diagrams are clearly defined in examples.
Ammonia is a compound of nitrogen and hydrogen with the formula NH 3.A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world ...
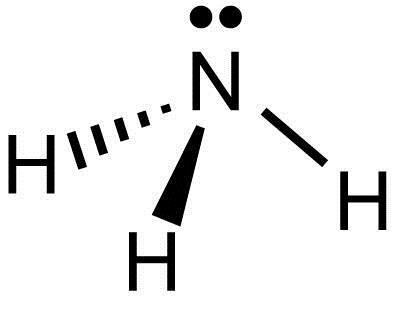
Yes, NH3 (Ammonia) molecule is polar in nature because of its asymmetrical shape ie; trigonal pyramidal structure, and the difference in electronegativities of N (3.04) and H (2.2). The charges over the nitrogen and hydrogen atoms are unequally distributed which results in a net dipole moment making NH3 (Ammonia) a polar molecule.
Draw the dot and cross diagram for ammonia, NH3. You need only show the electrons in the outer shells. When drawing a dot-and-cross diagram for an ionic compound it is usually acceptable to draw the outer electron shell of the metal ion without any electrons. 4 2018 97012318 2 (a) Nitrogen, N 2, is an inert gas that makes up 78% of the Earth ...
Hey everyone, welcome to the Mentor Center! In today's video, I draw out the Lewis dot structure for NH3, commonly known as ammonia.👍 Like 📽️ Subscribe ...
Draw dot-and-cross diagrams to represent the sharing of electron pairs to form single covalent bonds in simple molecules exemplified by but not restricted to H2 Cl2 H2O CH4 and HCl. A dot and cross diagram can show the bonding in a small molecule. For example the Lewis diagrams of two separate hydrogen atoms are as follows.
Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. by crator-avatar Jeff Bradbury 2.
So for Ammonium you need to identify the valence electrons for each of the elements. Nitrogen has 5 | Hydrogen has 1 * 4 | 4 Being the number of Hydrogen's.3 answers · 2 votes: Electrons of N is shared by H to complete its duplet and elctrons of H is shared by N to ...
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale...
![Draw an electron dot diagram to show the formation of each of the following compounds:Magnesium Chloride. [H = 1, C = 6, Mg = 12, Cl = 17] .](https://d1hhj0t1vdqi7c.cloudfront.net/v1/X2tnV2hFaU1EeFE=/sd/)
Draw an electron dot diagram to show the formation of each of the following compounds:magnesium chloride. [h = 1, c = 6, mg = 12, cl = 17] .
Lewis StructuresWe also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions, For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding ...
H2 Lewis Structure How To Draw The Dot Structure For H2 Chemical. NoName Dec 17, 2021 ...
Answer to which of the following represents the lewis dot diagram of ammonia nh3. Its highly soluble in water because its a polar substance. The lewis dot structure for nh3 starts with an n atom connected on three sides with a dash each to an h atom. Molecular structures are lewis dot structures example.
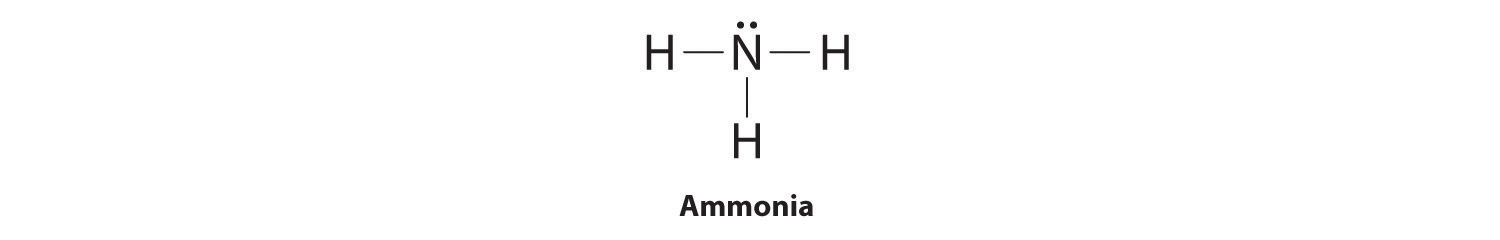
The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen.
Draw Electron Dot Structure For Ammonia Molecule - Drawing … Sep 01, 2021 · Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles Molecular Geometry Molecular Bond . Nh4 Lewis Structure How To Draw The Dot Structure For Nh4 Ammonium Science Chemistry Molecular Geometry Chemistry .
Please refer to the following Chapter 4 Carbon and Its Compound MCQ Questions Class 10 Science with solutions for all important topics in the chapter. Question. Carbon exists in the atmosphere in the form of. (a) Only carbon monoxide. (b) Carbon monoxide in traces and carbon dioxide. (c) Only carbon dioxide. (d) Coal.
Directions: Please highlight or bold the correct answer. 1. Which one of the following is most likely to be an ionic compound? a. CaCl2 b. CO2 c. CS2 d. SO2 2. Which one of the following is most likely to be an ionic compound? a. ClF3 b. FeCl3 c. NH3 d. PF3 3. […]
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Lewis Dot Diagram Of Nh3. Ammonia has the formula NH3. It's highly soluble in water because it's a polar substance. The Lewis structure for a molecule represents the total valence. The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Lewis Structures for NH3.
Lewis Structures: As valence electrons are significant to an atom's reactivity, it is essential to represent it by simple diagrams. Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. Hence, these structures are also known as electron dot diagrams.
As illustrated in Figure 3, a Lewis dot diagram shows only the valence electrons distributed between the covalently bonded atoms. An unshared electron pair or lone pair is represented by two paired dots. A short line (a single bond) is made of two electrons. Two short lines (a double bond) is made of four electrons.
In this paper, we emphasized the dual application of Cu-modified vertically aligned TiO2 nanotube arrays as photocatalyst and a relative humidity sensor. The TiO2 nanotube arrays were obtained by anodization of the titanium layer prepared using radio frequency magnetron sputtering (RFMS) and modified with different copper concentrations (0.5, 1, 1.5, and 2 M) by a wet-impregnation method.
Conjugate acid of SO 4-2 is HSO 4-. A conjugate acid is the product of a base gaining a proton for example the conjugate acid of ammonia NH3 is the ammonium ion NH4. Conjugate acid of NH3. So Conjugate acid of HSO 4- is H 2 SO 4. Similarly NH4 loses a proton to give conjugate base NH3. Conjugate acid is formed by adding an H ion to the given base.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electron s which for m 2 lone pairs. Since it is bonded to only one carbon atom, it must for m a double bond.. Answer (1 of 4): draw the lewis dot diagram for H2S.
H2 Lewis Structure How To Draw The Dot Structure For H2 Chemical. NoName Dec 18, 2021 ...
Lewis Dot Structure: Lewis structures are also known as Lewis dot structures or electron dot structures. These are basically diagrams with the element's symbol in the centre. The dots around it represent the valence electrons of the. element. ... In ammonia (NH3), the three hydrogen atoms share one electron each with the nitrogen atom and form ...
Perchlorate ion (ClO-4) have the following possible resonating structure. Cl-O bond order =7/4 =1.75. How do you find the bond order? If there are more than two atoms in the molecule, follow these steps to determine the bond order: Draw the Lewis structure. Count the total number of bonds. Count the number of bond groups between individual atoms.
The bonding pairs of electrons, or those that make bonds, shape the NH3 molecules. Using Lewis structure, we will understand electron geometry, polarity, and other properties of both polar and non-polar compounds. We must first determine the valence electrons that form dipole moments before we can establish the structure of ammonia molecules.
24 Oct 2020 — Which of the following is the correct Lewis structure for ammonia (NH3) respectively H H 1 H-N-H N-H * -N- H-N… Get the answers you need, ...2 answers · Top answer: The lewis structure of ammonia is the structure C.Given:The molecule ammonia NH_3To find:The ...
Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Subsequently, question is, what is the shape of nh3? trigonal pyramidal
Question: A molecule of ammonia, NH3 N H 3, and a molecule of methane, CH4 C H 4, each have 4 electron domains. a. Draw or describe the Lewis dot structures for each of these molecules. Is NH3 tetrahedral or trigonal pyramidal? Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair).
Electron Dot Structure of NH3 by Jeff Bradbury - February 17 - Lewis Electron Dot Structure for ammonia molecule NH3. A video explanation o...

Draw the lewis structures for the following molecules and ions : h2s, sicl4, bef2, co^2 - 3 and hcooh





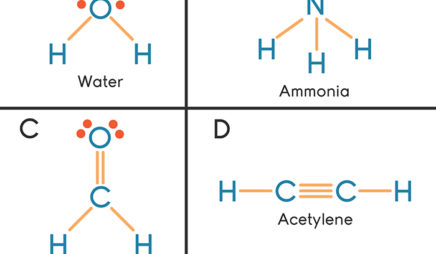












0 Response to "39 which of the following represents the lewis dot diagram of ammonia (nh3)?"
Post a Comment