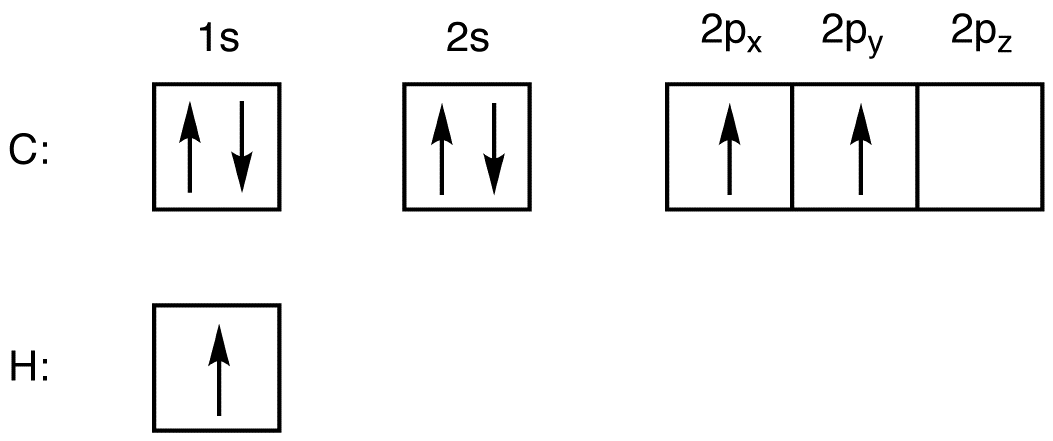
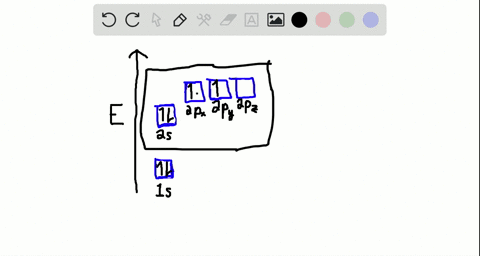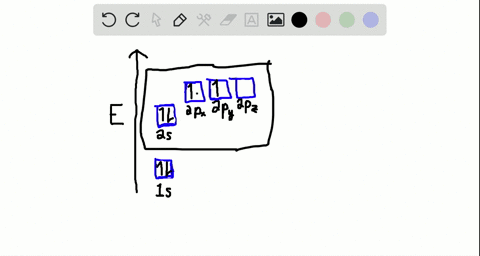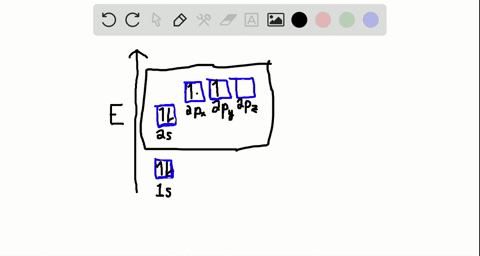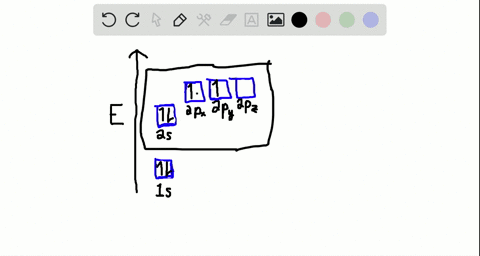
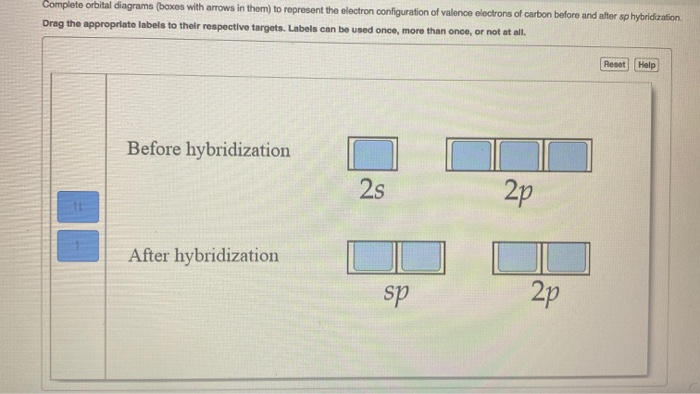
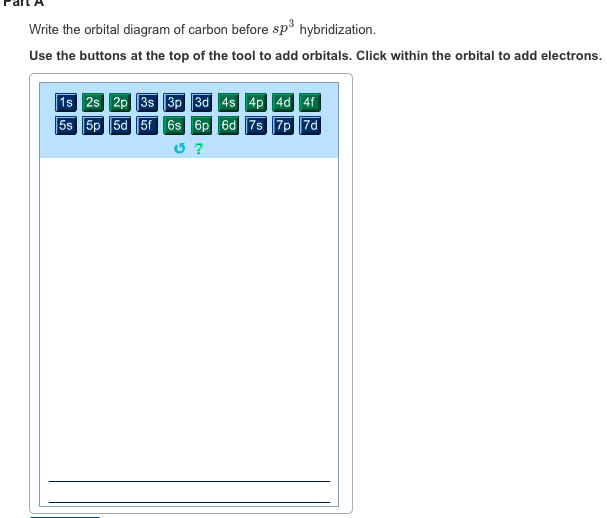
40 write the orbital diagram of carbon before sp3 hybridization.
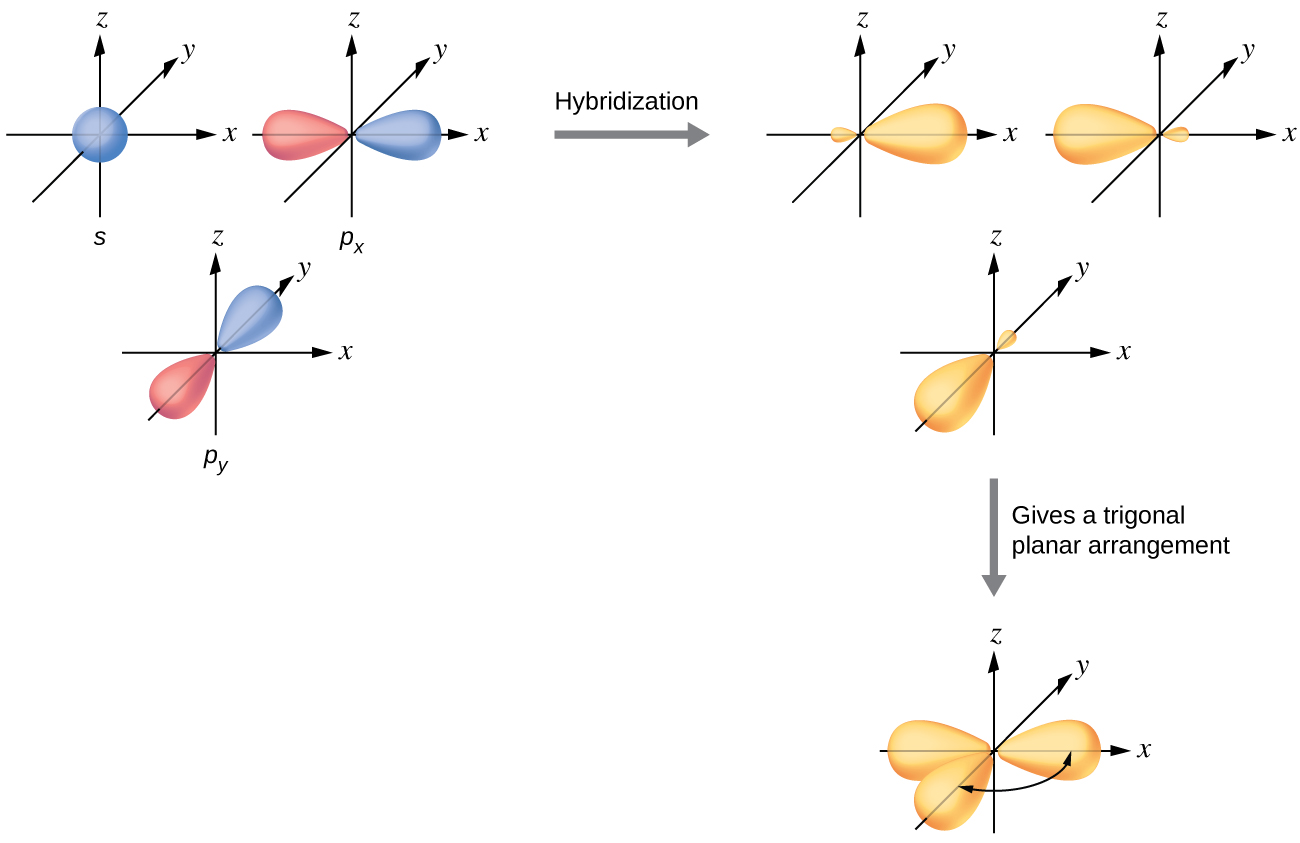
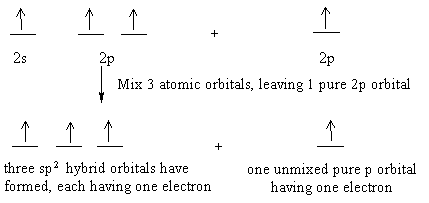
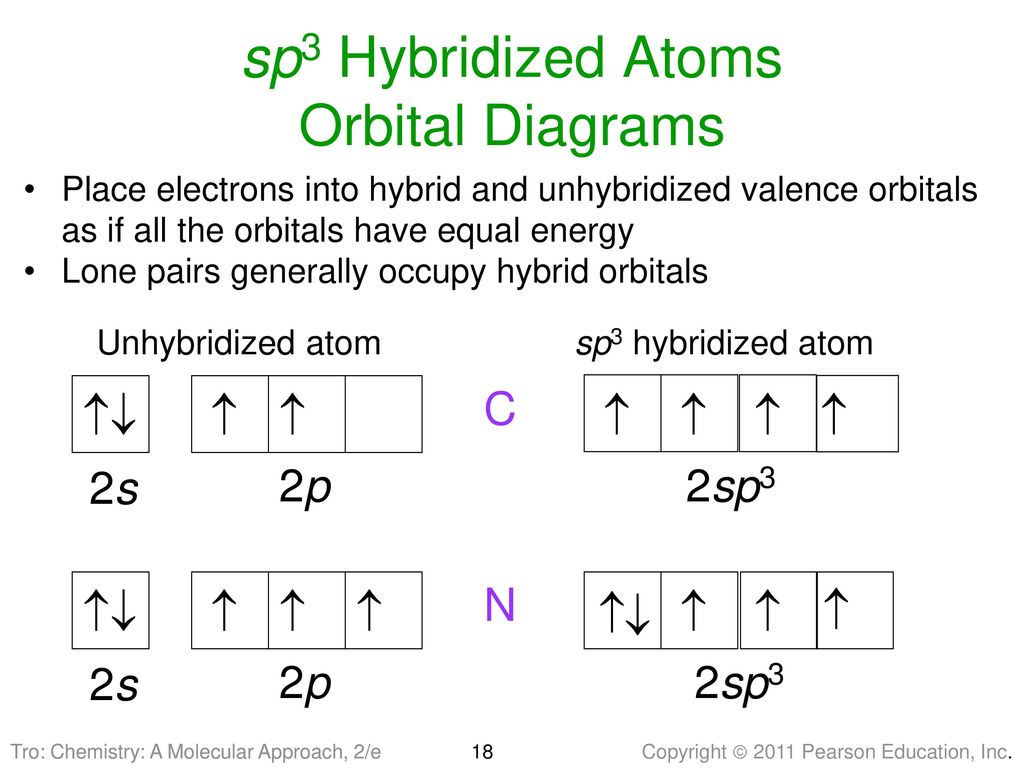
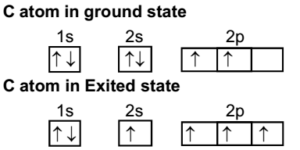
iii. In order to form four equivalent bonds with hydrogen, the 2s and 2p orbitals of C-atom undergo sp 3 hybridization. iv. One electron from the 2s orbital of carbon atom is excited to the 2pz orbital. Then the four orbitals 2s, px, py and pz mix and recast to form four new sp 3 hybrid orbitals having same shape and equal energy. They are ...
Clf2 lewis structure
Carbon is triple-bonded to nitrogen, and so there are one sigma and two pi bonds.As per rule, the first bond between any two atoms is a sigma bond, and the second/third bonds are pi bonds)This means two p orbitals are required to be left over after hybridization.2 pi bonds = 2 leftover p orbitals.As a result, one of carbons p orbitals is available to hybridize
Write the orbital diagram of carbon before sp3 hybridization.
C2cl4 lewis structure
Write the orbital diagram of carbon before sp3 hybridization; Alabama vs texas a&m 2016 score; Y cb/pb cr/pr; Which feature of a bond contract allows the issuer
Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
Write the orbital diagram of carbon before sp3 hybridization..
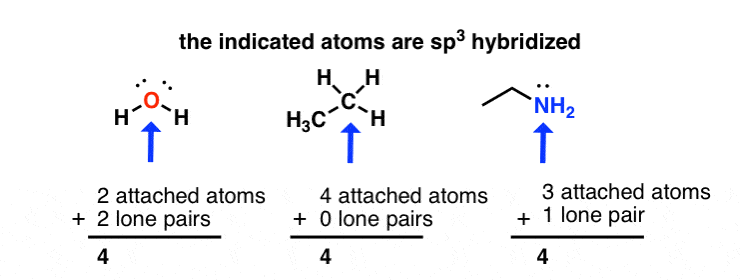
The bonds formed in Dichloromethane are covalent bonds. Central Carbon is hybridized as the molecule forms all the four bonds in the compound. An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds. Thus the hybridization of Carbon atom in CH2Cl2 is sp3. Molecular Geometry of Dichloromethane
The oxygen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. One of the sp 3 hybridized orbitals overlap with s orbitals from a hydrogen to form the O-H signma bonds. One of the sp3 hybridized orbitals overlap with an sp 3 hybridized orbital from carbon to form the C-O sigma bond.
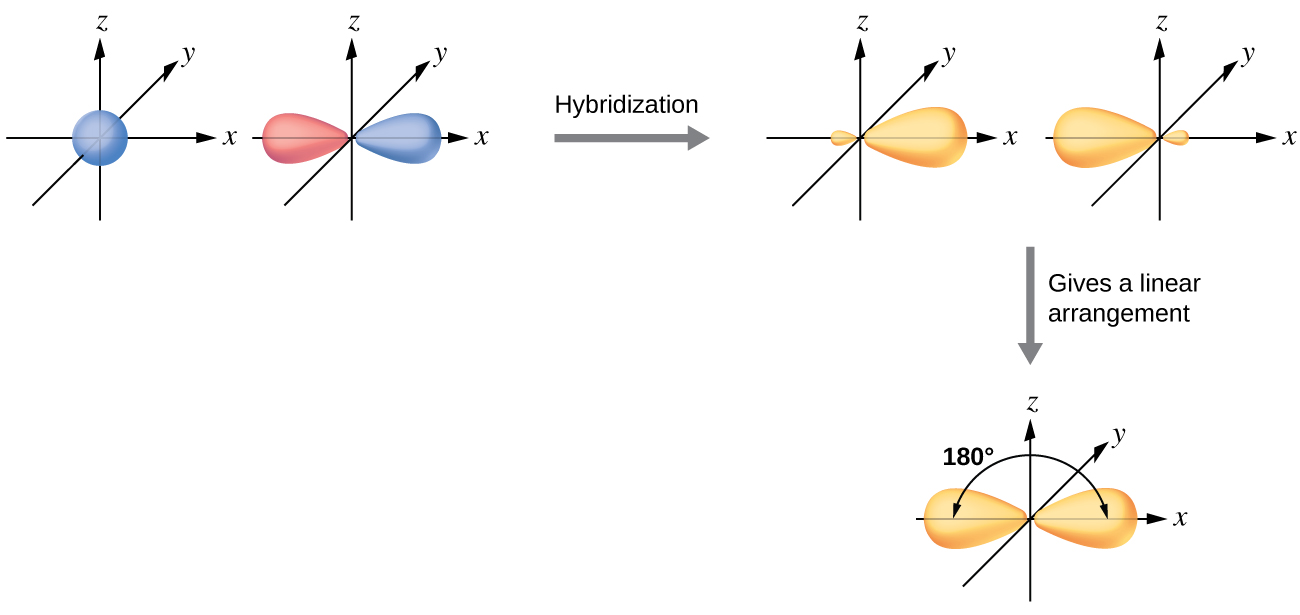
CO2 Hybridization. The hybridization of CO2 is Sp. The carbon atom is Sp hybridized and oxygen atoms are Sp2, making the overall molecule Sp hybridized. Hybridization can be understood by 2 methods, one by understanding the combination of the orbitals and 2nd by using a simple formula.
Academia.edu is a platform for academics to share research papers.
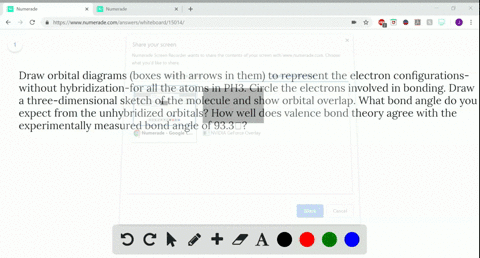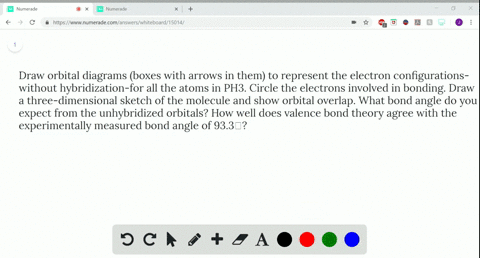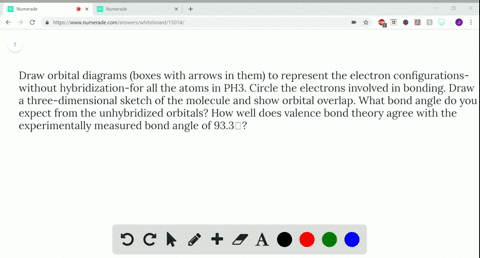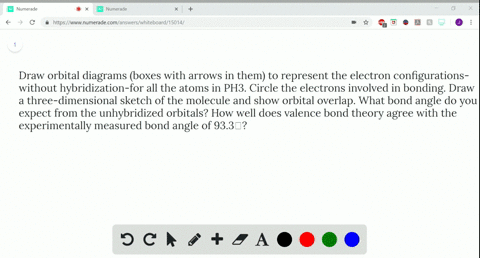
Q. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. Solved • Sep 30, 2021 Hybridization
When one S- orbital hybridize with three p - orbitals of an excited carbon atoms, SP3 hybridization is formed. After spreading out, the unpaired orbitals are aligned at 109o away from each other. They point to the cornets of a regular tetrahedron with carbon atom at the center of the tetrahedron.
The hybridization process involves taking atomic orbitals and mixing these into hybrid orbitals. For example, metal atoms can use appropriate d orbitals to overlap with the π*2p orbitals of the carbon monoxide molecule. (b) The magnetic properties of B2 are consistent with the π2p MOs being lower in energy than the σ2p MO.
Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ ...
During sp hybridization, one s and one p orbital of carbon combine to form two sp hybrid orbital s. Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like. So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital.
C(hybrid state): p O1(hybrid state): O2(hybrid state): sp2 sp2 p sp3 sp2 O1 sp2 sp2 sp2 sp3 sp2 sp3 sp3 sH C O2 sp3 sp2 s H 24 Intermolecular Forces: Hydrogen bond. 7. a) Carbon dioxide, CO2, has a triple triple point at 5.2 atm and -57oC and a critical point at 72.9 atm and 31oC. Sketch a phase diagram of CO2. Pressure (atm) 72.9 B C Solid ...
Are all sp3 orbitals degenerate? 1) An sp3 hybrid orbital may hold a lone pair of electrons. 2) may form a sigma bond by overlap with an orbital on another atom. 3) The sp3 hybrid orbitals are degenerate. Is sp3 a sigma or pi? The number of orbitals taking part in hybridization is the number of sigma bonds made around the central atom.
Carbon, the central atom, is sp2 hybridized. The S atom is the central atom for SF2. The hybridization of the S atom is sp hybridized. It has two orbitals 180 degrees apart. The central atom undergoes sp3d2 hybridization. SP hybridization sp3 would be the hybridization The central atom of PCl5 is P. In this case, phosphorous has an
1 Answer to Divalent carbon species called carbenes care capable of fleeting existence. For example, methylene: CH2, is the simplest carbene. The two unshared electrons in methylene can be either spin-paired in a single orbital or unpaired in different orbital. Predict the type of hybridization you expect...
Example: Hybridization of CO2. sp2 Hybridization: When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. Example: Hybridization of graphite. sp3 Hybridization: When the carbon atom is bonded to four other atoms.
1, carbon 2. Write hybridization schemes for the formation of sp, sp2, and sp3,sp3d, and sp3d2 hybrid orbitals; predict the geometric shapes of molecules in terms of the pure and hybrid orbitals used in bonding. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules.
It explains how to write the orbital diagram nPractice. 3 (which also contains a table of atomic orbital energies). 1. Br2 2+ should be paramagnetic because when you remove the 2 electrons, the 3pi-x and 3pi-y will be unpaired, like Shreyesi said. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; …
Write the orbital diagram of carbon before sp3 hybridization; I told you so lyrics keith urban; Doris day if i give my heart to you; What they want lyrics; Documentary now! season 2 episode 3; Are arboreal folivores of the australian temperate forests
The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals.
Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron ...
What is the hybridization of Cycloalkanes? Cycloalkanes are composed of sp3 hybridized carbon and hydrogen atoms connected by sigma bonds. However, unlike linear hydrocarbons, which can achieve a more stable tetrahedral configuration around each carbon atom in the backbone, the bond angles in cycloalkanes are constrained, producing ring strain.
Learn about our Editorial Process. Updated on February 01, 2021. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104.
In carbon dioxide molecule, oxygen also hybridizes its orbital s to form three sp 2 hybrid orbital s. The p orbital in oxygen remains unchanged and is mainly used to form a pi bond. However, out of the three sp hybrid orbital s, only one will be used to form a bond with the carbon atom. ... 39 write the orbital diagram of carbon before sp3 ...
03.03.2017 · Aromatic vs Antiaromatic vs Non Aromatic Practice Exercises. Our last post in this series on aromaticity went through the 4 conditions a molecule must fulfill in order to be aromatic.. First, it must be cyclic Second, every atom around the ring must have an available p-orbital; Third, the number of electrons in the pi system must be 2, 6, 10, 14, 18, or a higher number in …
The highlighted atoms have sp2 - sp3 hybridization.The highlighted atoms are carbon and nitrogen as shown.. The structure of Xanthine is shown in the image attached to this answer.The indicated atoms are Carbon and nitrogen as shown. Let us remember that hybridization has to do with the mixing of atomic orbitals to form appropriate hybrid orbitals that are suitable for overlap with orbitals of ...
An sp3 hybrid orbital is unsymmetrical in shape, having one small and one large lobe. The four sp3 hybrid orbitals of a group are equivalent in shape and energy. Which of the following options correctly describe sp hybrid orbitals? Select all that apply. The energy of an sp hybrid orbital lies between the energies of the original s and p orbital that were mixed. Each sp hybrid orbital …
In carbon dioxide molecule, oxygen also hybridizes its orbital s to form three sp 2 hybrid orbital s. The p orbital in oxygen remains unchanged and is mainly used to form a pi bond. However, out of the three sp hybrid orbital s, only one will be used to form a bond with the carbon atom. Part B. Draw the orbital diagram for the ion Co2+.Use the buttons at the top of the tool to add orbital s in ...
Answer (1 of 2): In writing the symbols for elements, a capital letter or a capital letter followed by a lower case letter is used. There ate no elements with the symbols Bf and Sf. The problem refers to compounds. So lets start by assuming you meant BFx and SFy where xand y are subscripts. Then...
Here the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise two of the orbitals. Halogen bonds ("XB" for short) are ubiquitous in biochemistry and materials chemistry. Demo: balloon VESPR. Orbital hybridization can determine how many bonds an atom can form and the shape of molecules. Berthier, in ...
There are several types of hybridization like SP3, SP2, SP. BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double bond between the Boron and only three σ bonds are formed per Boron atom. The atomic S - orbitals and P - orbitals in Boron outer shell mix to form three equivalent SP2 hybrid ...
How to determine Hybridization - s, sp, sp2, and sp3 - Organic Chemistry Hybridization is a section of bonding in chemistry. In examinations, you may have some questions such as identifying hybridization of atoms and determine which hybridized orbitals are attached to make bonds.
Vor 2 Tagen · Diagram orbital hybridization using orbital nota ion. hybridization state of carbon indicated by the arrow in the structure of DEET shown below? A) sp B) sp2 C) sp3 D) sp3d E) sp3d2 Ans: B 27. It actually bonds. C02 Differentiate between localized and delocalized electrons within a structure. 5 mole Methanethiol (CH3SH) has been implicated in the …
Enter the email address you signed up with and we'll email you a reset link.
Carbon atoms 1 and 3 are sp² hybridised while carbon 2 is sp hybridized. The two unhybridised orbitals of carbon 2 overlap sidewise with unhybridised p orbitals of carbon 1 and 3 to form π bonds. Click 👉 Atomic structure Part 3
which partial orbital diagram correctly shows the hybrid orbitals and electron distribution of the N atom in the molecule NBr3. C sp3 . valence bond theory describes a single covalent bond as the _____ of orbitals form two atoms to form a shared space, which is occupied by _____ electrons. overlap 2. why is the hybridization model necessary to explain the bonding in a molecule …







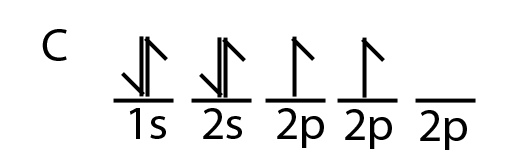


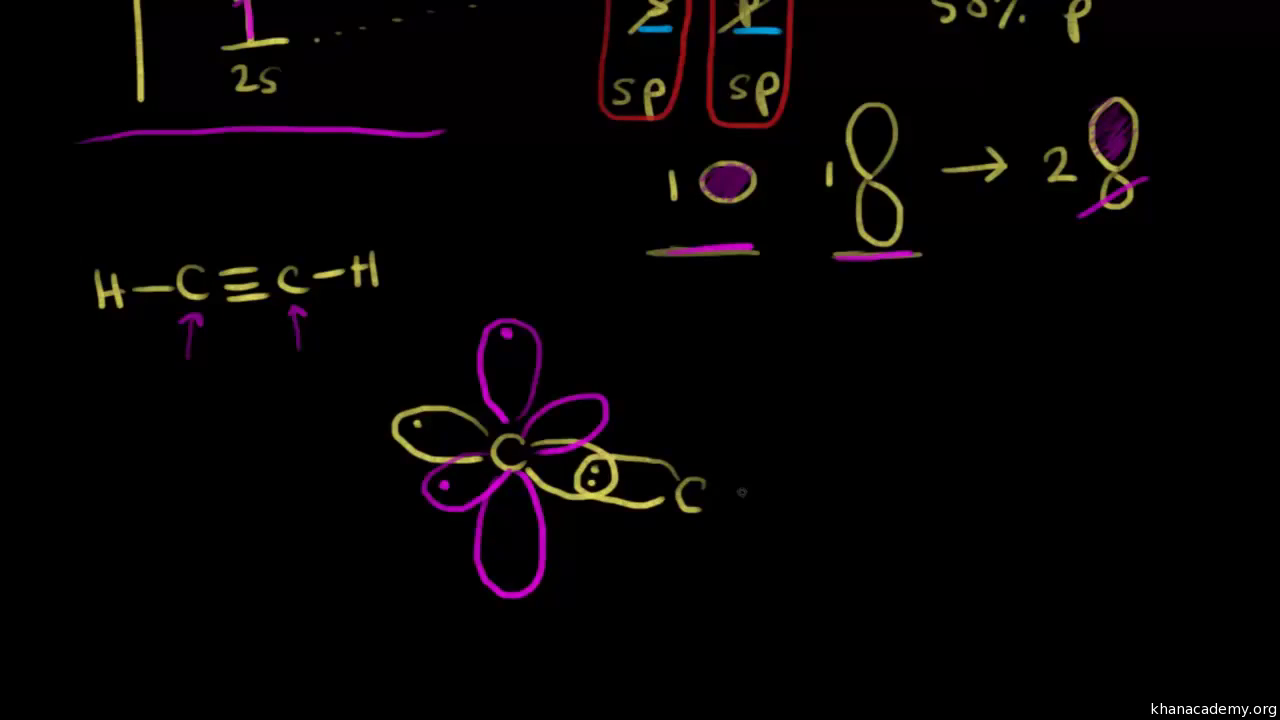









0 Response to "40 write the orbital diagram of carbon before sp3 hybridization."
Post a Comment