41 bohr diagram for neon
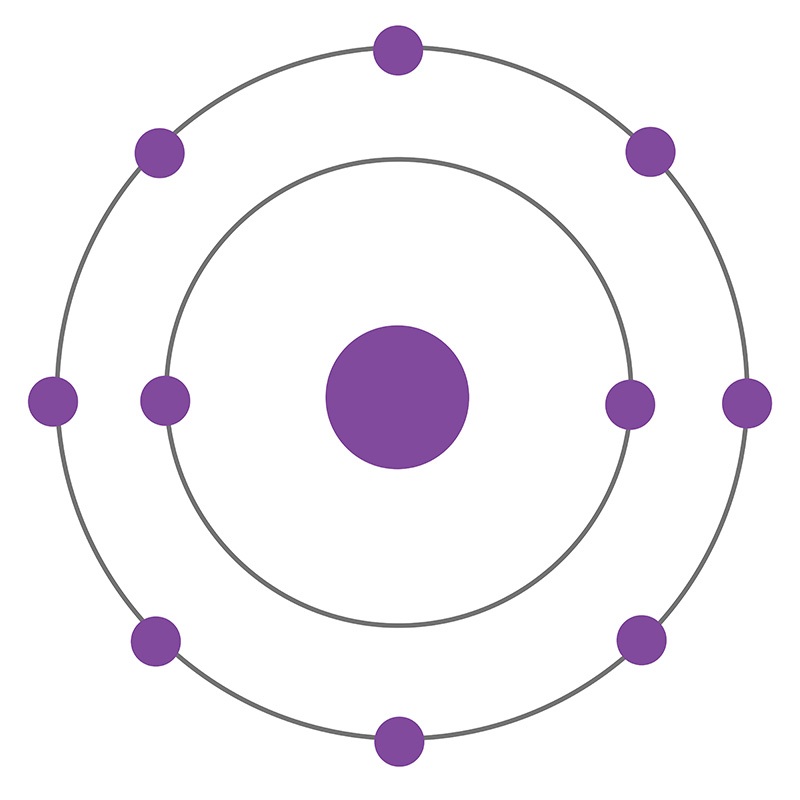
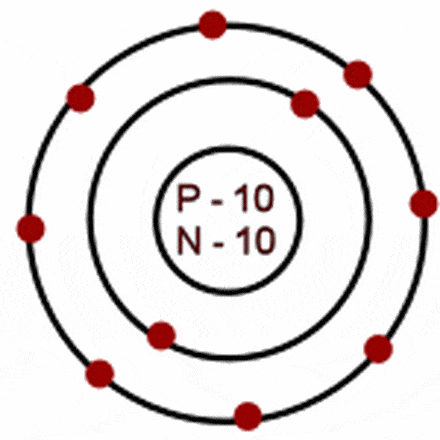
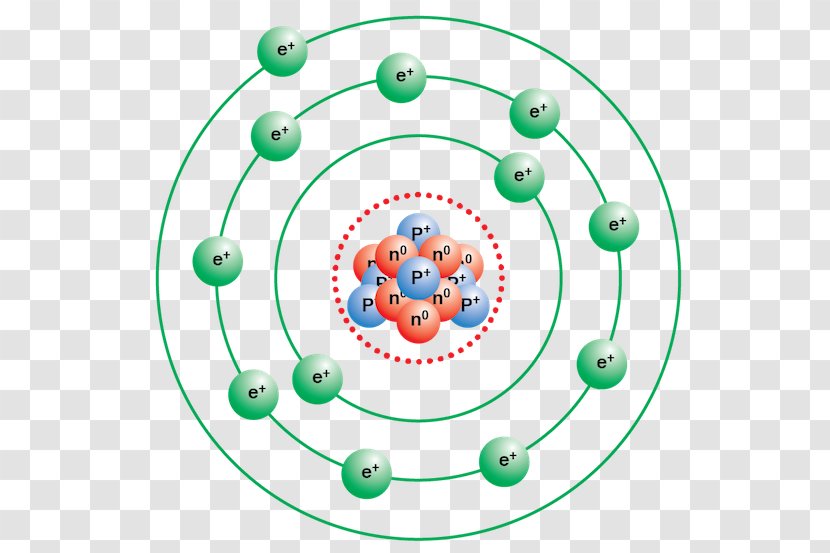
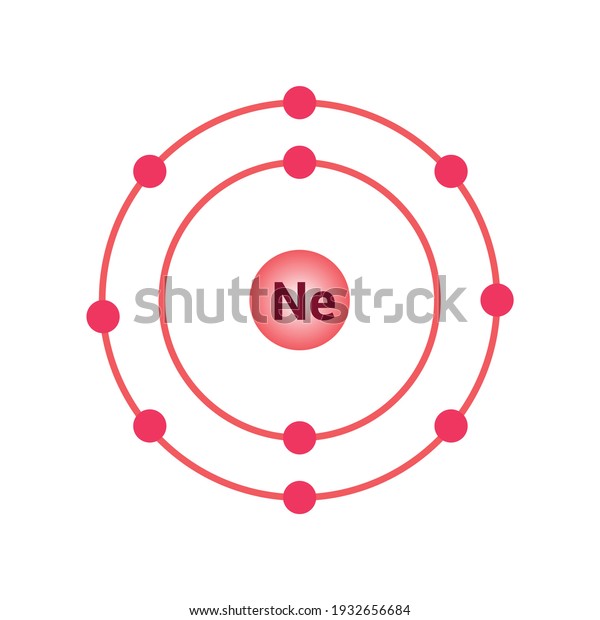
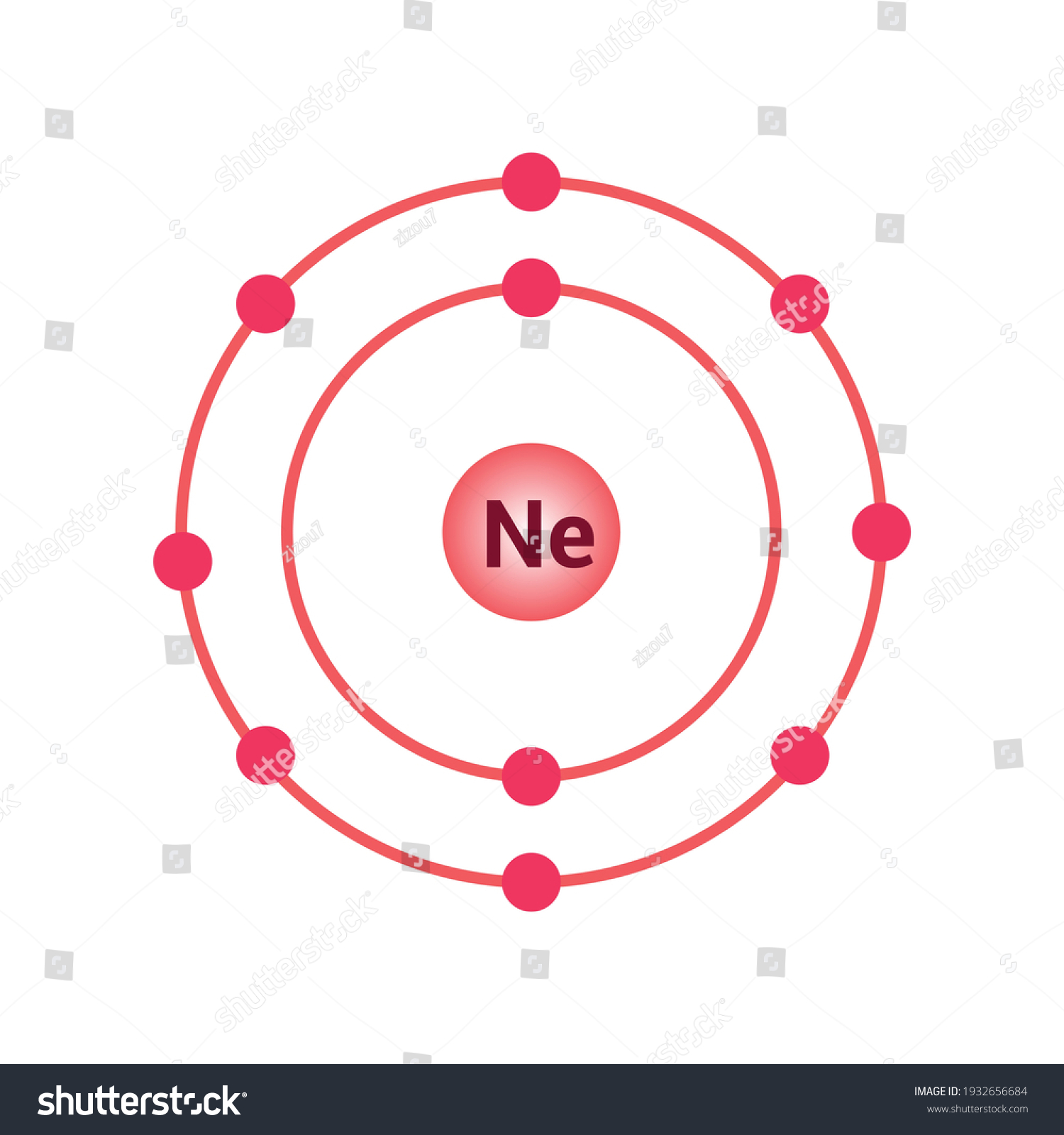
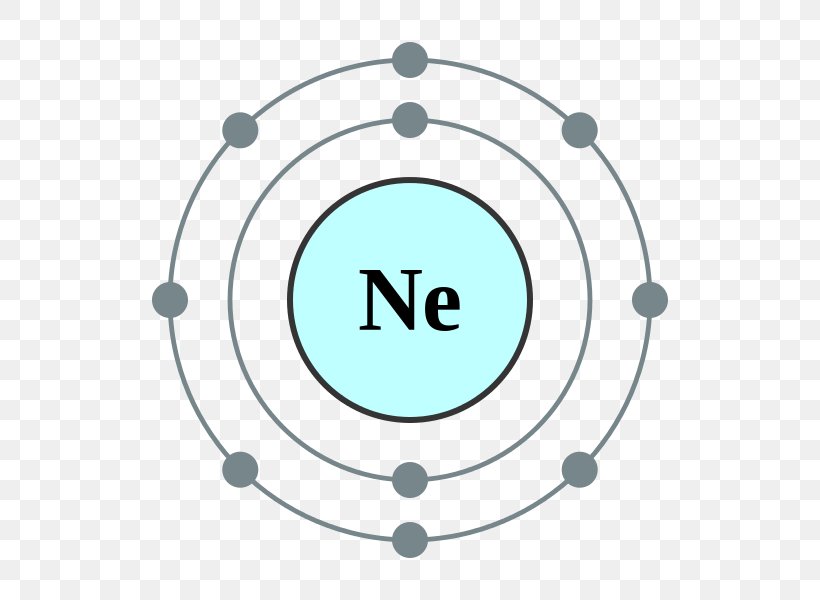
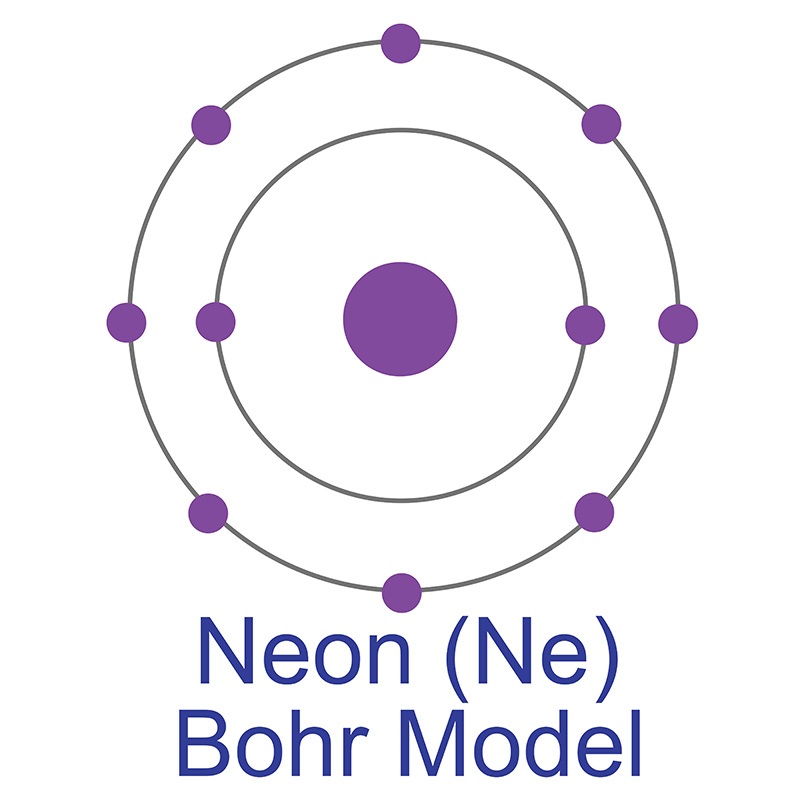
Oct 16, 2018 · Neon Bohr Diagram. To confirm the third electron of neon enters the orbit of two According to the Bohr hydrogen-like model, the radius. Two electron shells surrounding the nucleus, containing 2 electrons in the n=1 shell and 8 electrons in the n=2 shell. Bohr's model of the atom. Bohr model Neon atomic orbits.
Neon has 2 electrons in its first shell and 8 in its secondCheck me out: http://www.chemistnate.com
The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom, singly ionized helium, and doubly ionized lithium, but it includes positronium and Rydberg states of any atom where one electron is far away from everything else.
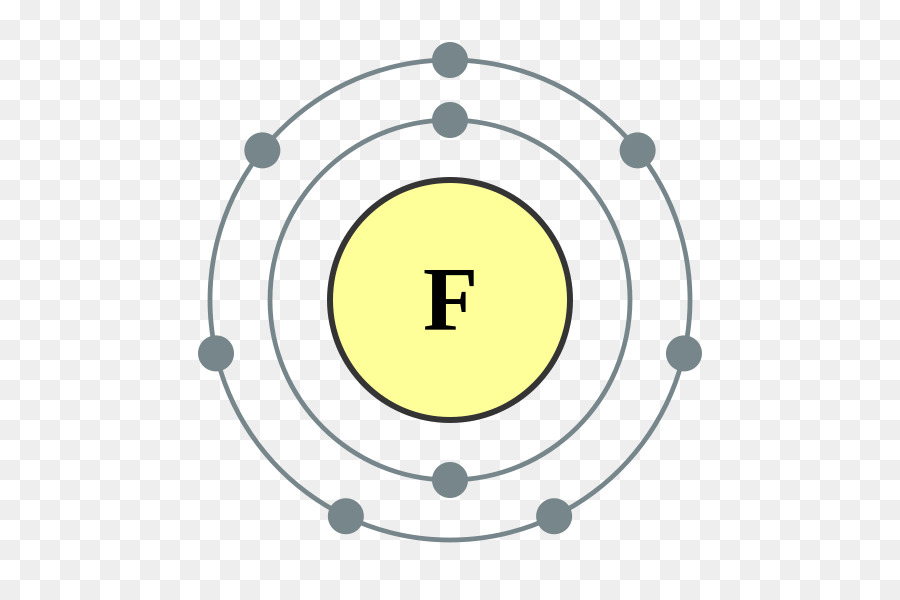
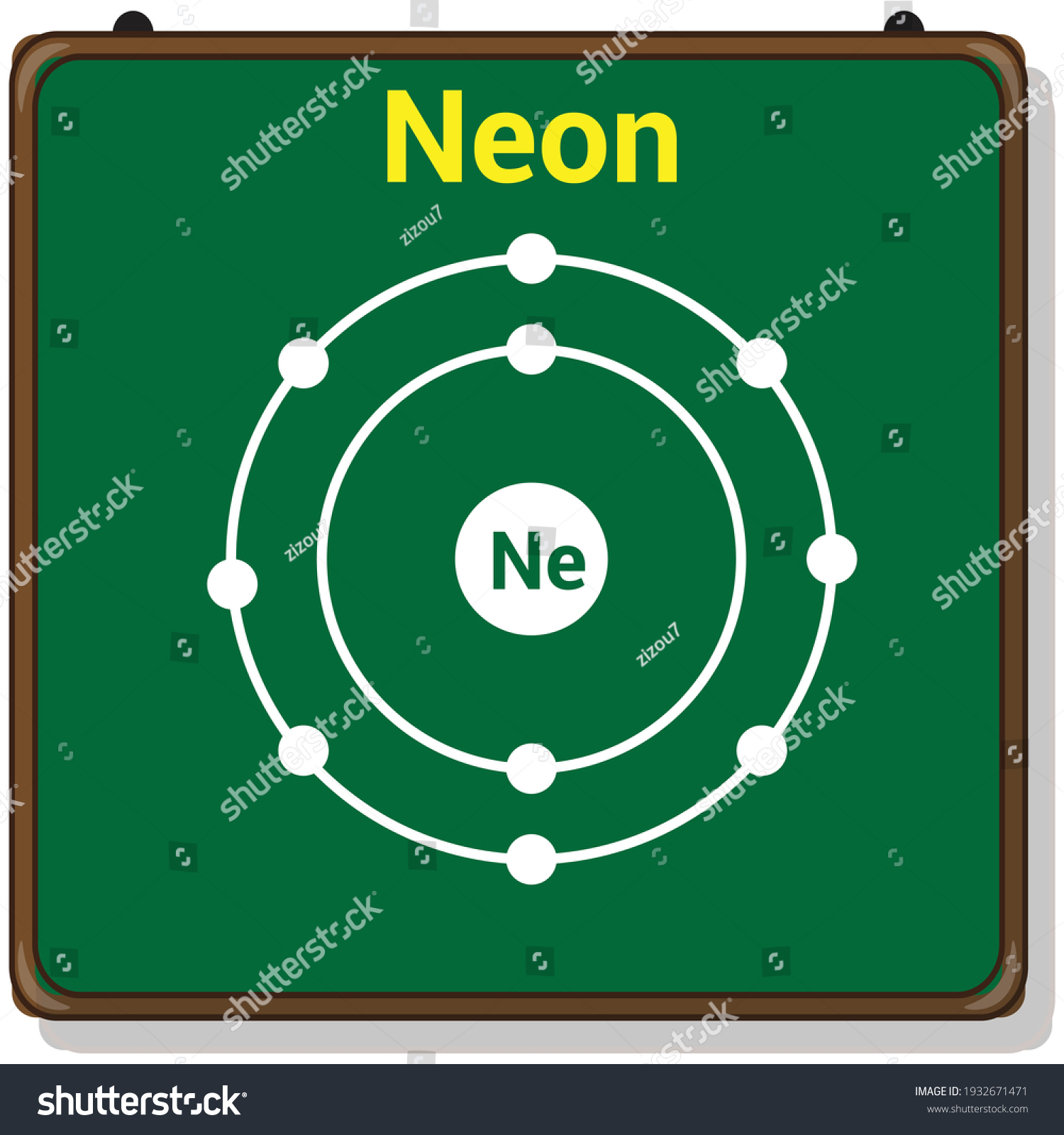
Bohr diagram for neon
Bohr Model Questions and Answers. Get help with your Bohr model homework. Access the answers to hundreds of Bohr model questions that are explained in …
Dec 23, 2021 · Beside above, what is the Bohr diagram for neon? Two electron shells surrounding the nucleus, containing 2 electrons in the n=1 shell and 8 electrons in the n=2 shell. Bohr’s model of the atom described the atom as a series of energy levels called principle quantum shells, at progressively greater distance from the nucleus.
Notes: Remember that Metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive “cations”. Nonmetals tend to gain electrons, filling up their current energy levels, becoming larger, forming negative “anions”.
Bohr diagram for neon.
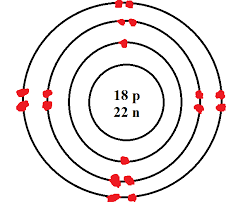
Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a neon atom, fluorine ion, and a magnesium ion? ALI e ec Levels 4. What would you expect to see with the arrangement of …
Bohr’s diagram of Neon has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Neon atom are its valence electrons because it is the outermost shell that also called the valence shell.
This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, ... Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are …
Bohr Model of Neon Neon Atom Model, Atom Model Project, Bohr Model, Neon. Visit . Andrew's model of a neon atom Science Projects For Kids, Science. In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by The second orbit allows eight electrons, and when it is full the atom is neon, again inert.
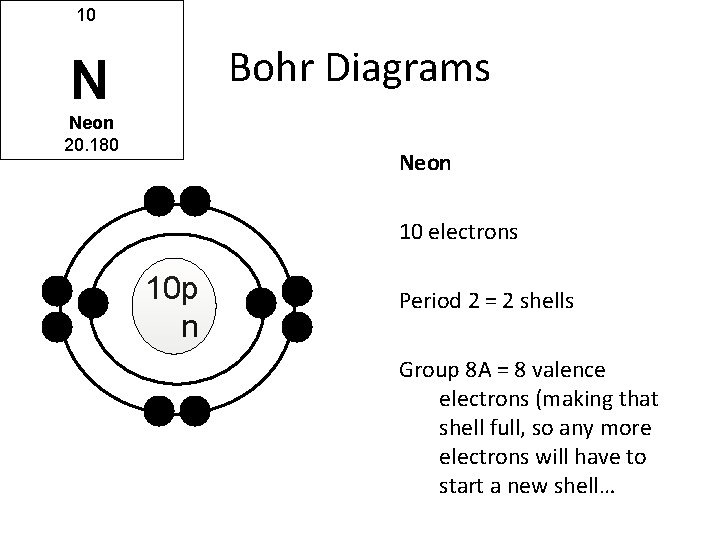
24.09.2019 · In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we’ll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons . You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom. In the model for the …
01.09.2021 · Similarly, neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Theoretically, they would be more energetically stable if they followed the octet rule and had eight. Bohr Rutherford Diagram For Nitrogen . Bohr diagrams show electrons orbiting the nucleus of …
Bohr’s diagram of Neon has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Neon atom are its valence electrons because it is the outermost shell that also called the valence shell. The L-shell or outer shell of the Neon Bohr model contains 8 electrons, therefore, the number of valence ...
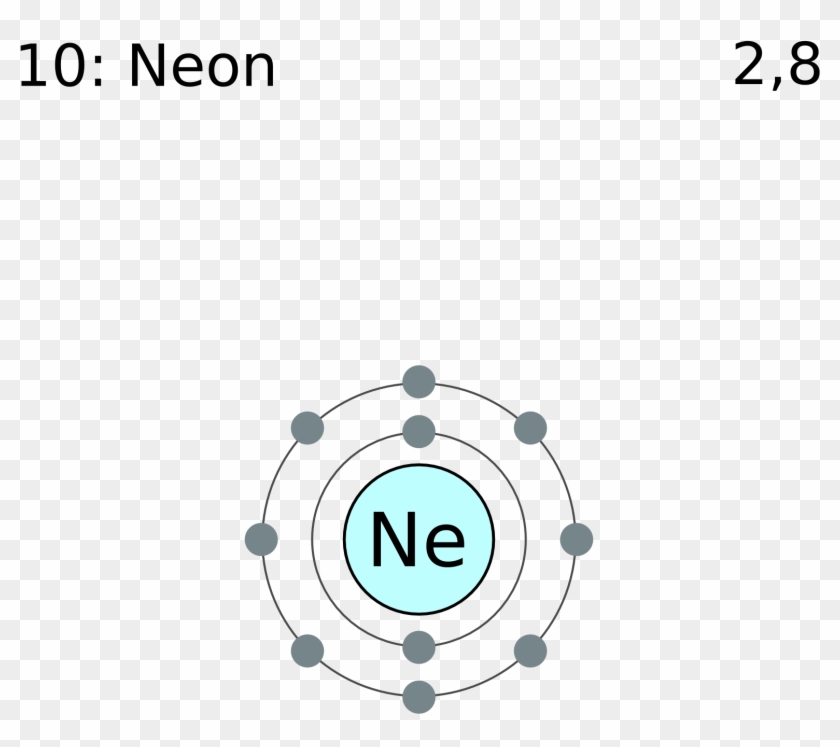
15.08.2020 · Bohr diagrams. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially-filled valence shells and gain or lose electrons to achieve a ...
Oct 01, 2021 · 15 Neon Bohr Diagram. A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913. This is a model that can be used to predict the emission spectrum of a neon. 1 st energy level can hold 2 electrons 2 nd energy level can.
07.03.2021 · Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8 ...














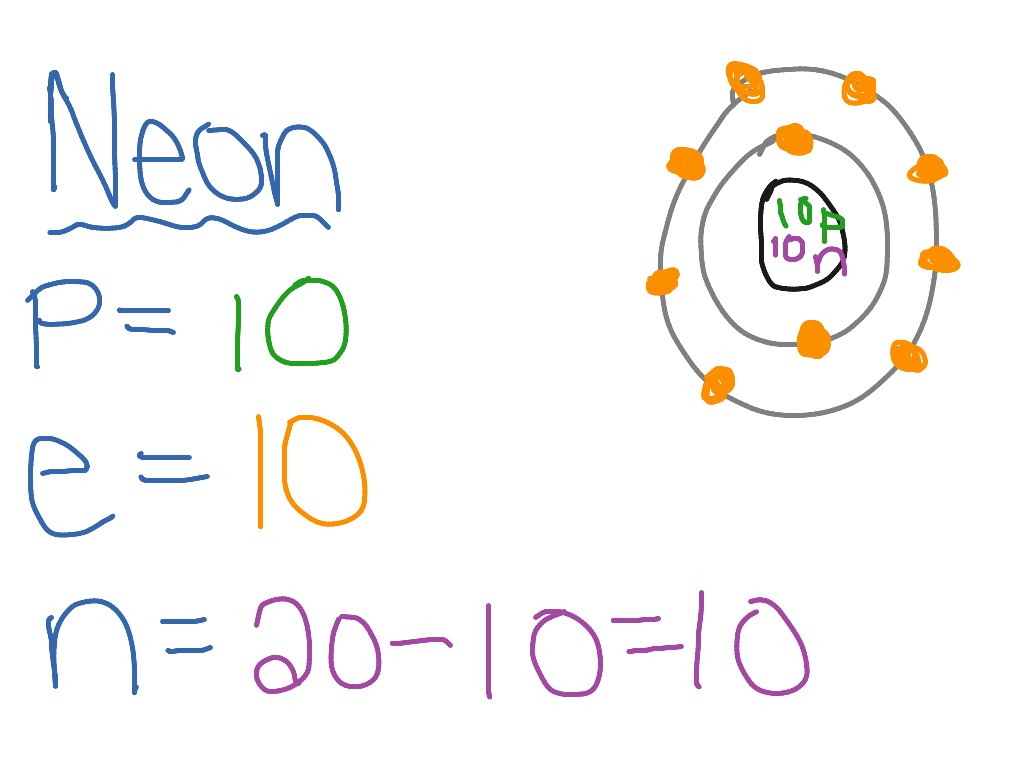









0 Response to "41 bohr diagram for neon"
Post a Comment