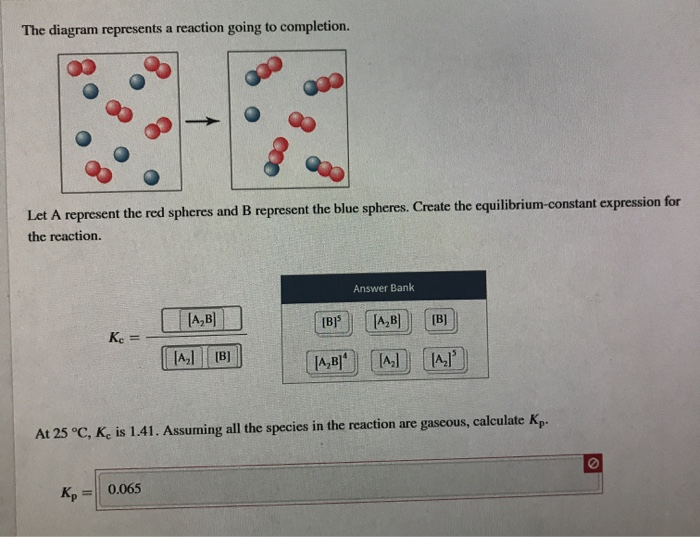
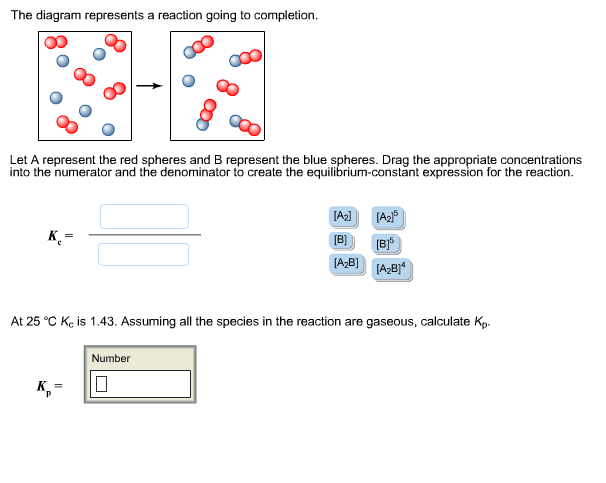
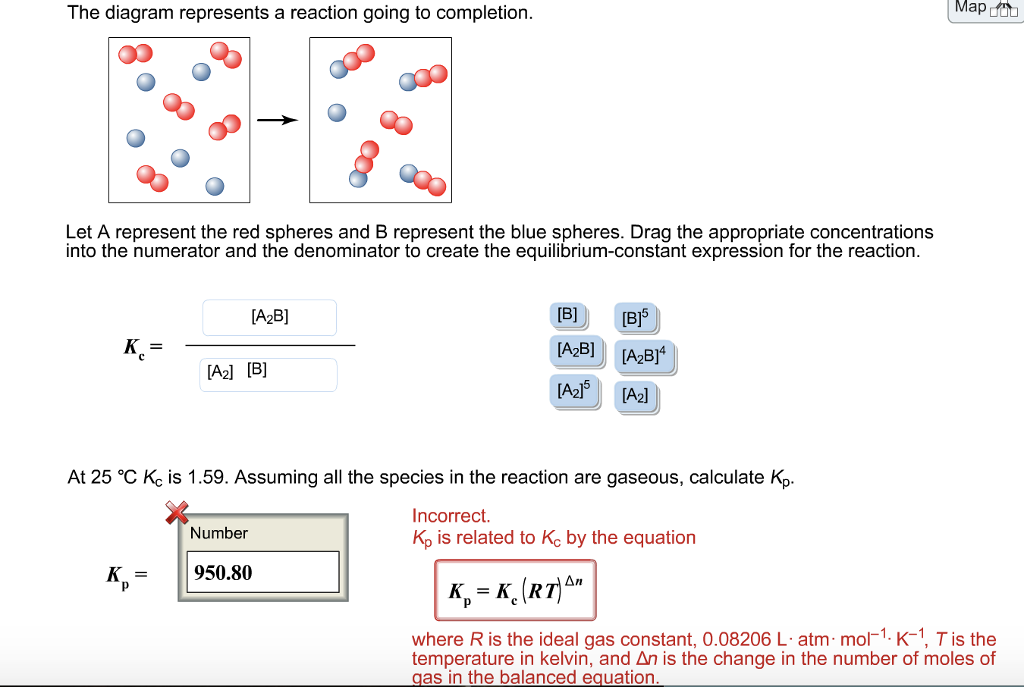
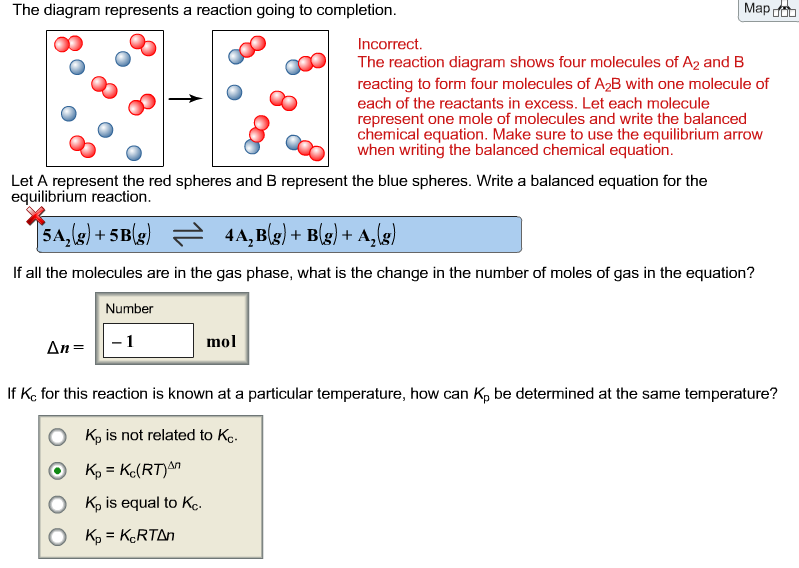
41 the diagram represents a reaction going to completion.
Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol, and the volume of the box is 1.0 L. Assuming that all of the molecules are in the gas phase, calculate n, the change in the number of gas molecules that accompanies the reaction.
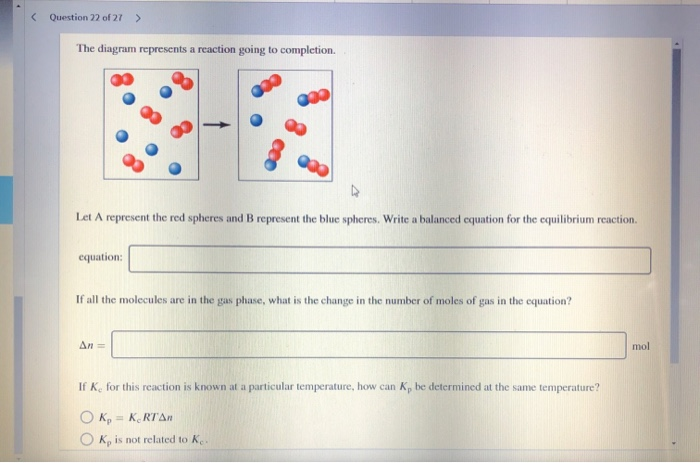
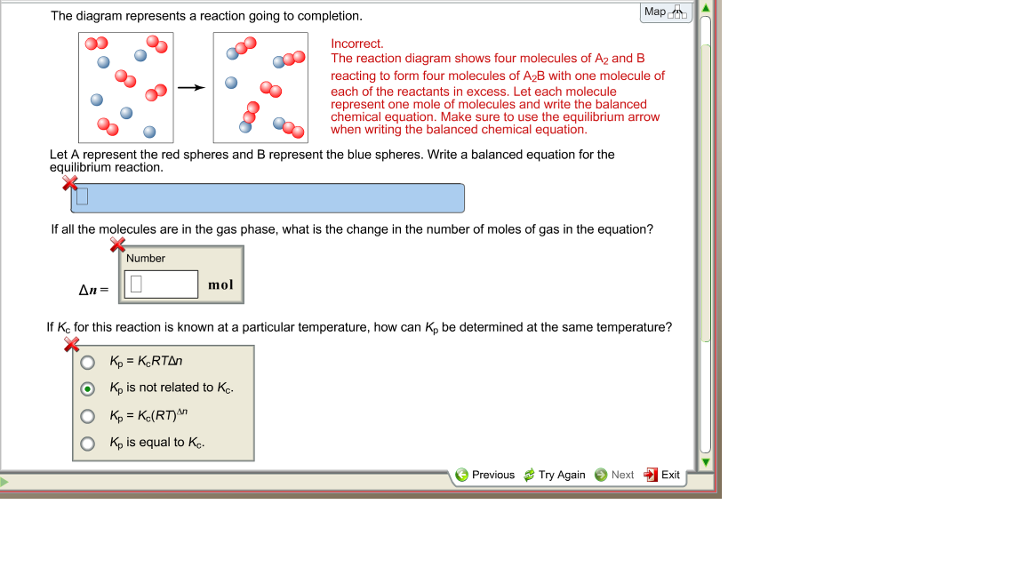
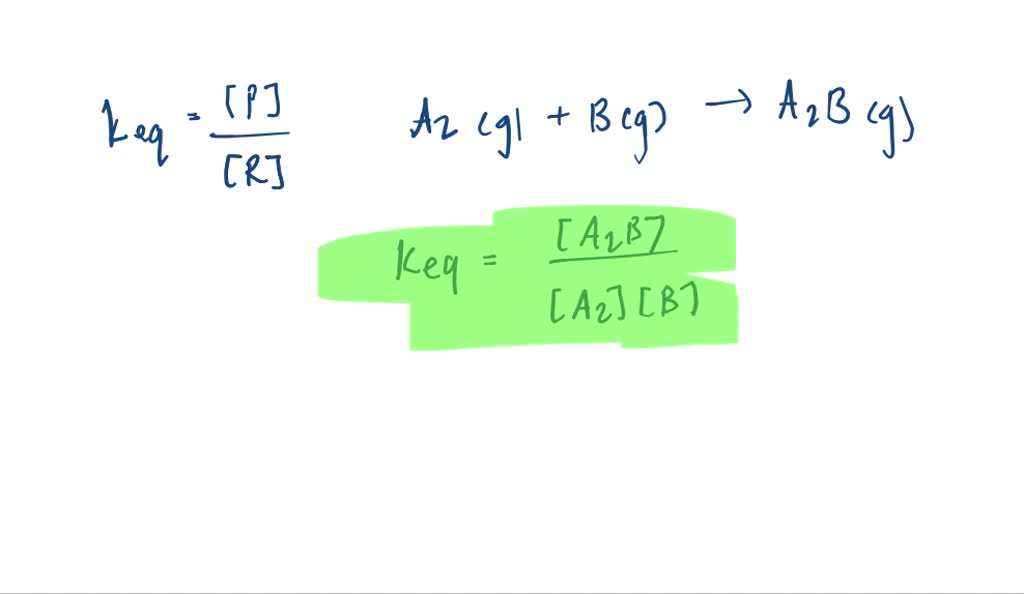
The diagram represents a reaction going to completion Let A represent the red spheres and B represent the blue spheres. Write a balanced equation for the equilibrium reaction equation: If all the molecules are in the gas phase, what is the change in the number of moles of gas in the equation? mol Δη = equation: If all the molecules are in the ...

The diagram represents a reaction going to completion.
The diagram represents a reaction going to completion. Let A represent the red spheres and B represent the blue spheres. Drag the appropriate concentrations into the numerator and the denominator to create the equilibrium-constant expression for the reaction. At 25 °C Kc is 1.59. Assuming all the species in the reaction are gaseous, calculate Kp. 15.4 The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reac-tion. (b) Write the equilibrium-constant expression for the re- The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. Express your answer as a chemical equation.
The diagram represents a reaction going to completion.. The diagram represents a reaction going to completion. Let A represent the red spheres and B represent the blue spheres. Write a balanced equation for the equilibrium reaction. The diagram represents a reaction going to completion. Let A represent the red spheres and B represent the blue spheres. Write a balanced equation for the equilibrium reaction. If all the molecules are in the gas phase, what is the change in the number of moles of gas in the equation? Delta_n = If K_c for this reaction is known at a particular. The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol, and the volume of the box is 1.0 L. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. (b) Write the equilibrium constant expression for the reaction. (c) Calculate the value of Kc. The following diagram represents a reaction shown going to completion. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. (b) Write the equilibrium- constant expression for the reaction. (c) Assuming that all of the molecules are in the gas phase, calculate (n, the change in the number of gas molecules ...
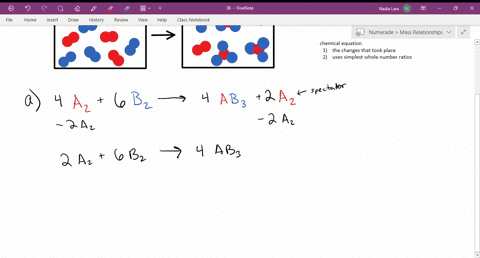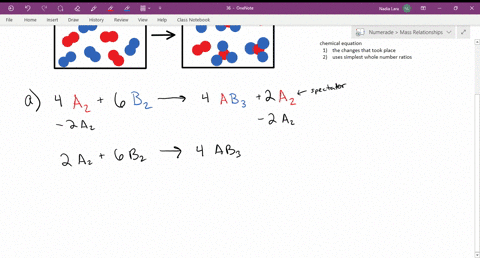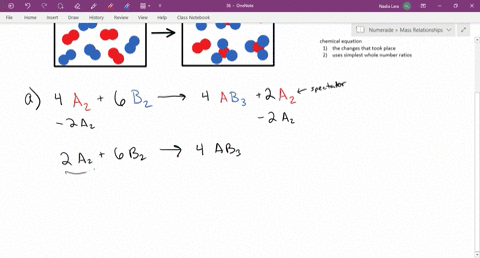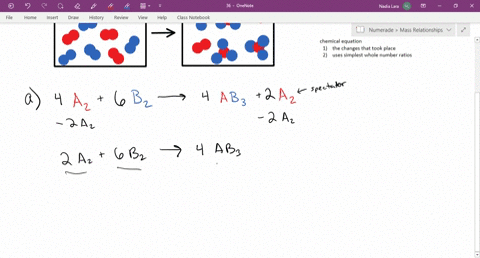
CHM 116--Chapter 15. The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol, and the volume of the box is 1.0 L. You may want to reference (Pages 628 - 632) Section 15.2 while completing this problem. Letting A = red spheres and B = blue spheres, write a balanced equation for the ... Problem Details. The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol, and the volume of the box is 1.0 L. Calculate the value of K c. A reaction reaches equilibrium: • once the rate of the forward reaction equals the rate of the reverse reaction • once at equilibrium there is no net change in the concentration of reactants or products. The sequence from left to right represents the system as time passes. Let's analyze each diagram: This side represents the substances that we have after the reaction has taken place. They are called the products. REACTANTS (before the reaction) → PRODUCTS (after the reaction) Do you see how the atoms have rearranged? This means a chemical reaction has taken place. Label the diagram with 'reactants' and 'product'.
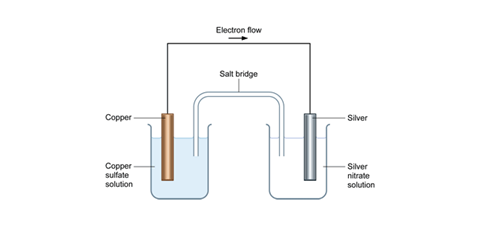
The cell reaction is the overall reaction which takes place in the cell, written on the assumption that the right hand electrode is the cathode, i.e. assuming that the spontaneous reaction is the one in which reduction is occurring in the right-hand compartment. (It is simple, from a knowledge of the standard potentials of the two electrodes ... Remove the ammonia (NH 3) as it forms. Lower the pressure. Lower the temperature. Increase the temperature. Add more hydrogen (H 2) Add a catalyst. For the reaction. H 2 (g) + I 2 (g) ⇋ 2HI (g) at equilibrium, in a 1 litre container, the amount of H 2 is 1 mole, the amount of I 2 is 2 moles & the amount of HI is 2 moles. the equilibrium constant K EQ for a particular reaction is given by the following expression KQ. It's equal to the product concentration divided by the reactant concentration. And so what we have is a violence chemical equation. To consider a two as a gas at B As a gas gives us a two B. And so our K equilibrium expression is equal to A to B, divided by a two, multiplied by the concentration of B. The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction.(b) Write the equilibrium-constant expression for the reaction.(c) Calculate the value of K c. (d) Assuming that all of the molecules are in the gas ...
The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. Part A Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. Part B Choose the equilibrium-constant expression for the reaction. Part C Calculate the value ...
Chapter 15 A. The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol, and the volume of the box is 1.0 L. Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. Nice work!
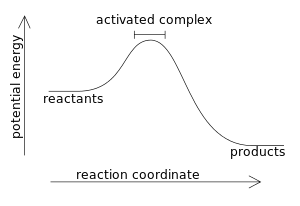
An enthalpy diagram allows us to easily see details of a chemical reaction. By knowing how to draw and label an enthalpy diagram we can see what the starting energy level is, how much energy is ...
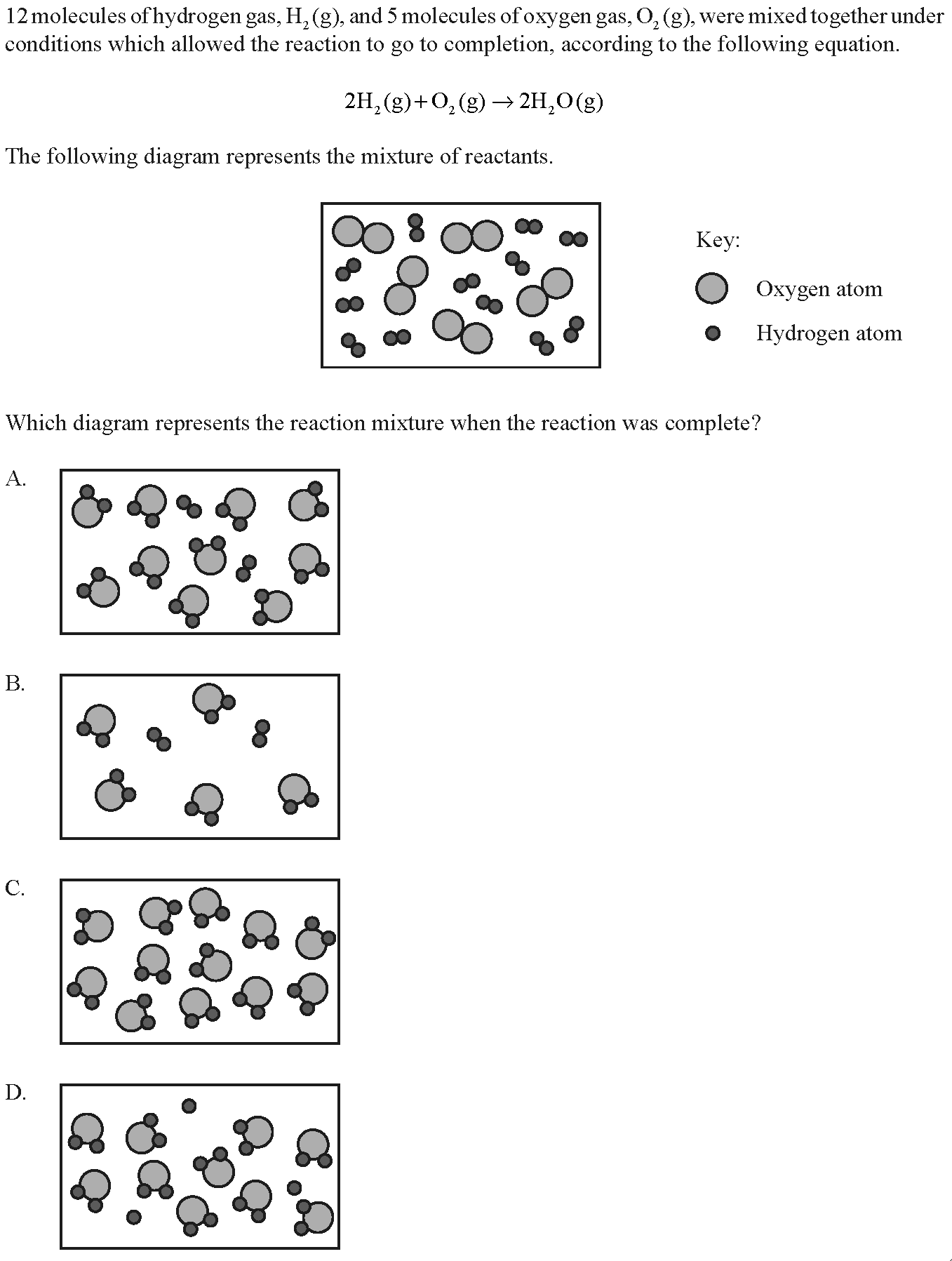
12 molecules of hydrogen gas, H2(g) and 5 molecules of oxygen gas, O2(g), were mixed together under conditions which allowed the reaction to go to completion, according to the following equation. 2H2(g) + O2(g)--> 2H2O. Which diagram represents the reaction mixture when the reaction was complete?
The following diagram represents a reaction shown going to completion.(a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction.(b) Write the equilibrium- constant expression for the reaction.(c) Assuming that all of the molecules are in the gas phase, calculate (n, the change in the number of gas molecules that accompanies the reaction.(d) How can you ...
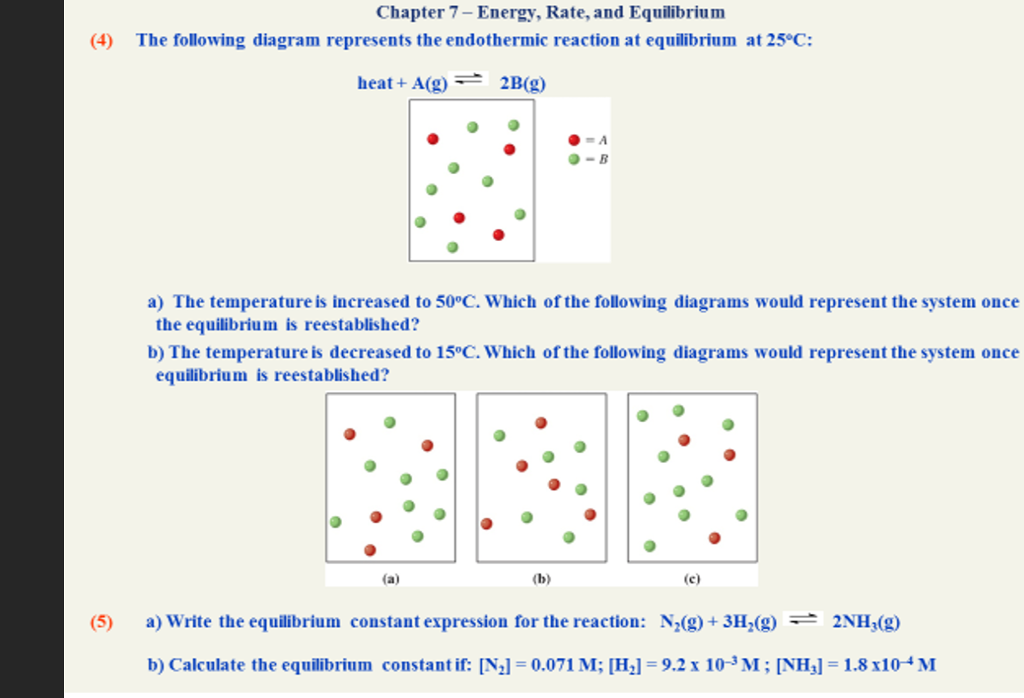
The diagram above represents H 2(g) and N 2(g) in a closed container. Which of the following diagrams would represent the results if the reaction shown below were to proceed as far as possible? N 2(g) + 3 H 2(g) à 2 NH 3(g) Gelder/McGaw 9 2. The diagram above represents a mixture of NO
The diagram represents a reaction going to completion. Let AA represent the red spheres and BB represent the blue spheres. Write a balanced equation for the equilibrium reaction. equation: If all the molecules are in the gas phase, what is the change in the number of moles of gas in the equation? Δ𝑛=Δn= molmol
The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for the reaction. (c) Is the diagram consistent with the law of conservation of mass?
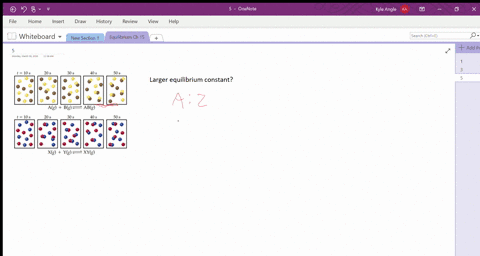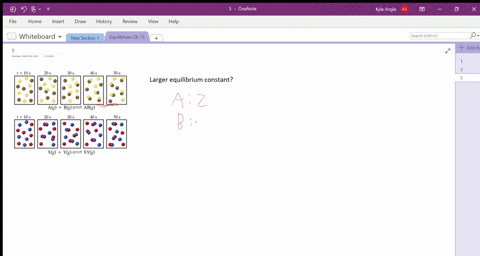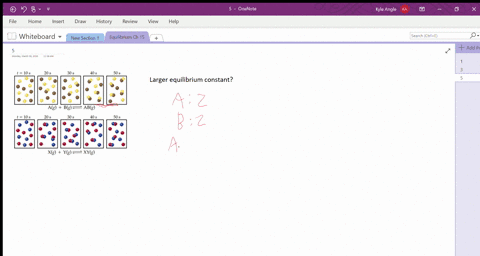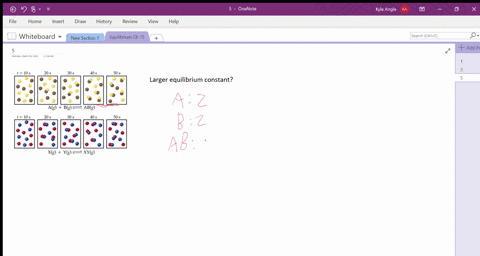
the diagram represents a reaction going to completion. In the reactants there are 5 single blue spheres and 5 pairs of 2 red spheres. In the products, there is one single blue sphere 1 pair of 2 red spheres and 4 groups of one blue and two red spheres. Let A represent the red spheres and B represent the blue spheres.
15.3 The following diagram represents an equilibrium mixture produced for a reaction of the type A + X AX. If the volume is 1 L, is K greater or smaller than 1? [Section 15.2] 15.4 The following diagram represents a reaction shown going to completion. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction.
Select the balanced equation that represents the combustion of octane, C8H18. ... Use the diagram below to answer the question: ... What do we call a reaction that goes to completion? Irreversible reaction. In the reversible equation of N2(g) + 3 H2(g) --> 2NH3 (g)
The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. Express your answer as a chemical equation.
15.4 The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reac-tion. (b) Write the equilibrium-constant expression for the re-
The diagram represents a reaction going to completion. Let A represent the red spheres and B represent the blue spheres. Drag the appropriate concentrations into the numerator and the denominator to create the equilibrium-constant expression for the reaction. At 25 °C Kc is 1.59. Assuming all the species in the reaction are gaseous, calculate Kp.


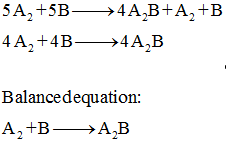











0 Response to "41 the diagram represents a reaction going to completion."
Post a Comment