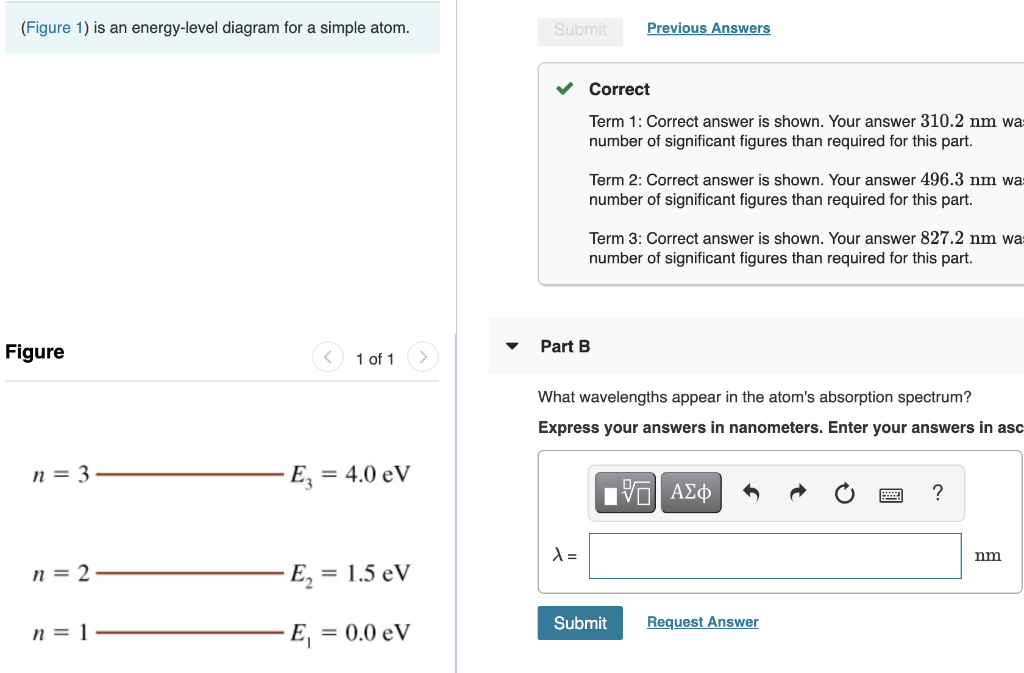
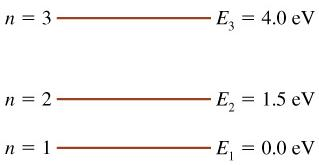
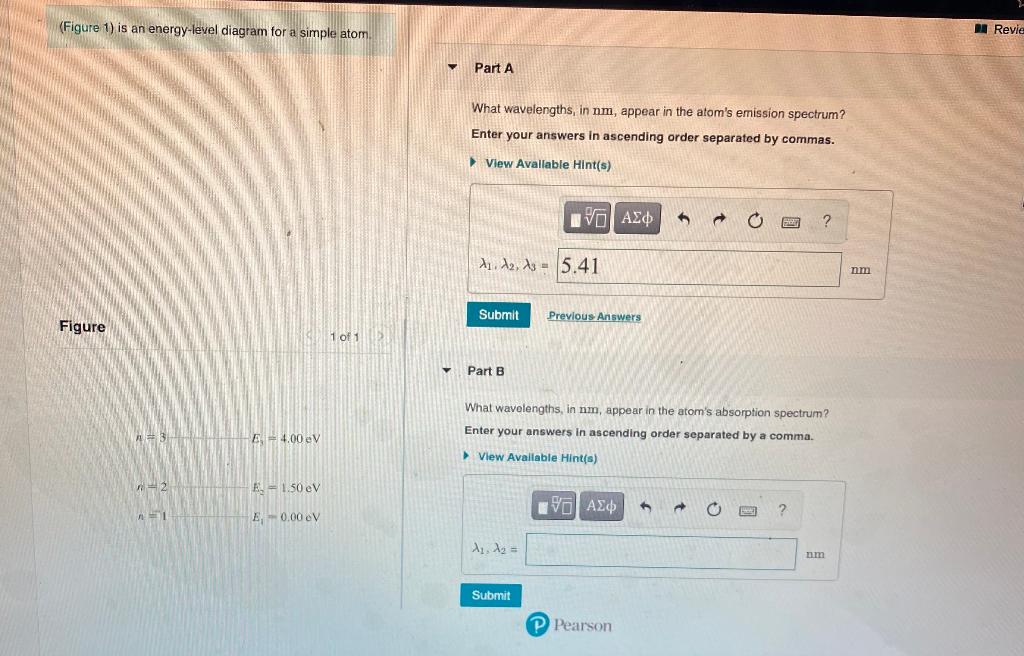
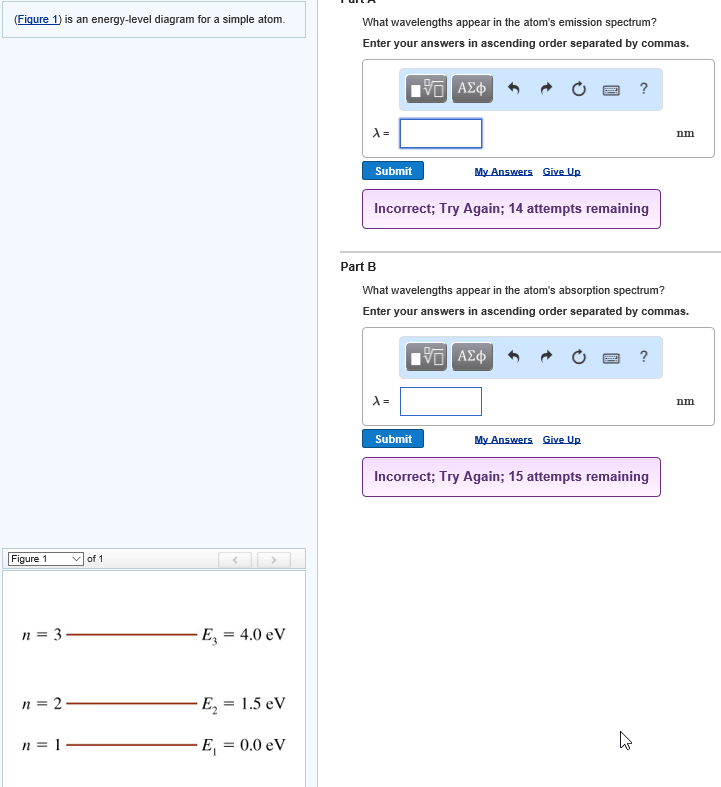
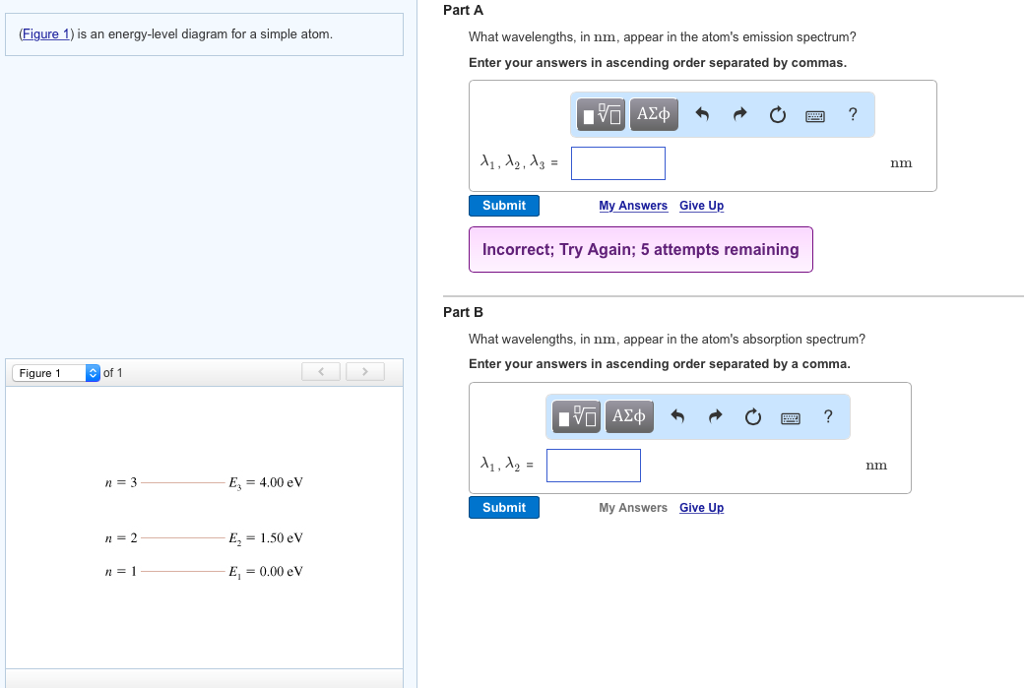
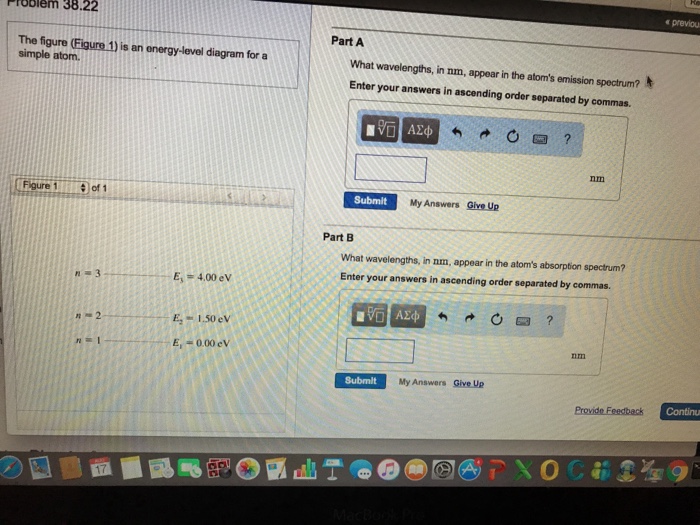
41 the figure is an energy-level diagram for a simple atom. (figure 1)
The chapter on atoms, molecules, and ions introduced the basic idea of ... of the binding energy curve (Figure 2) and is one of the most stable nuclides.
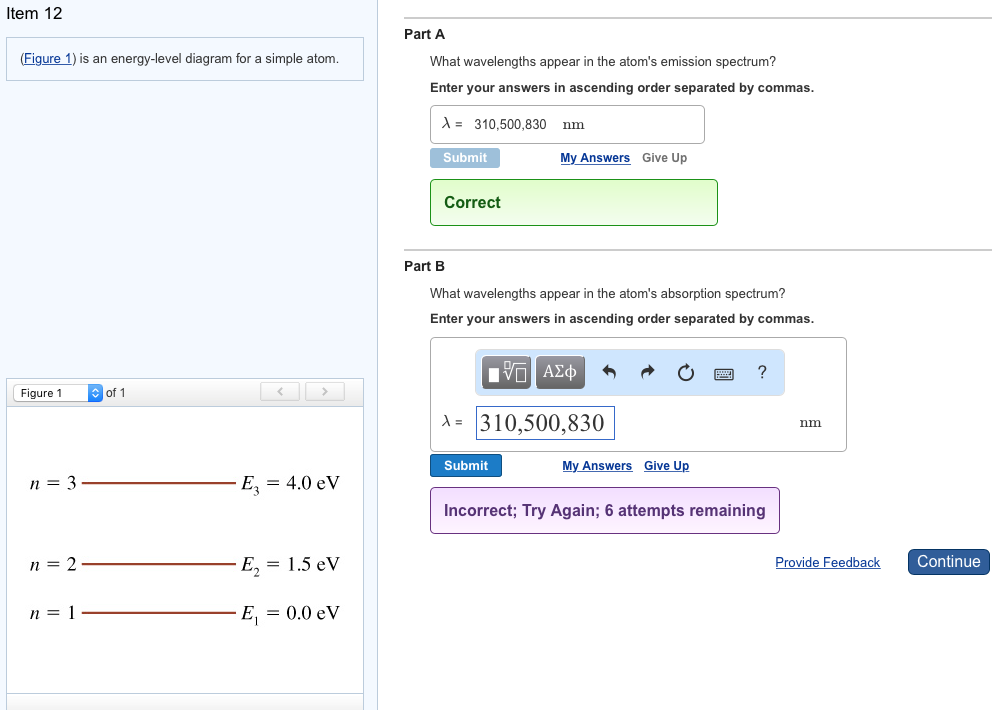
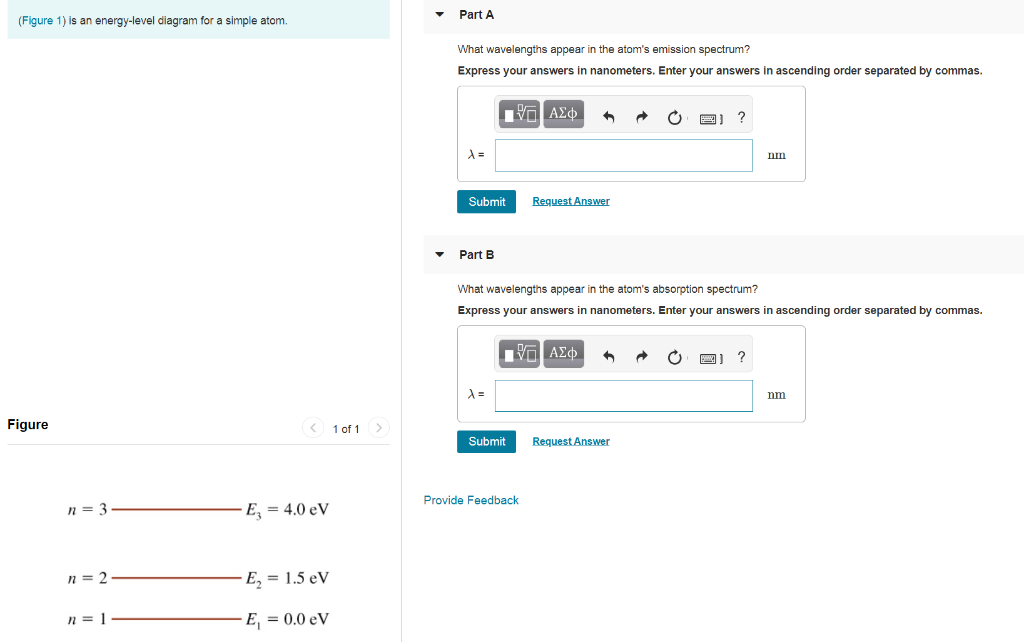
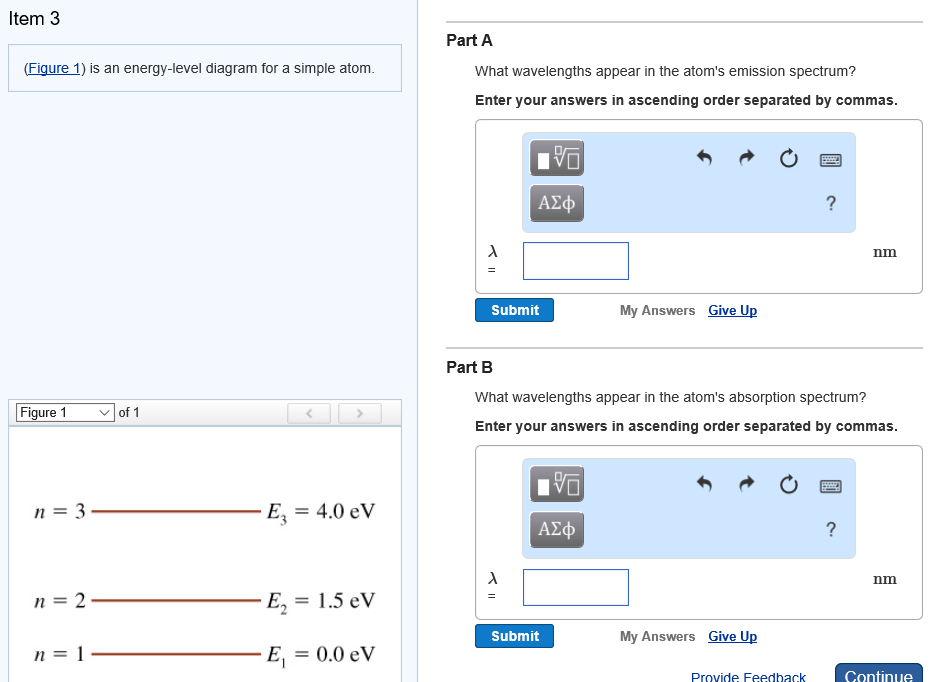
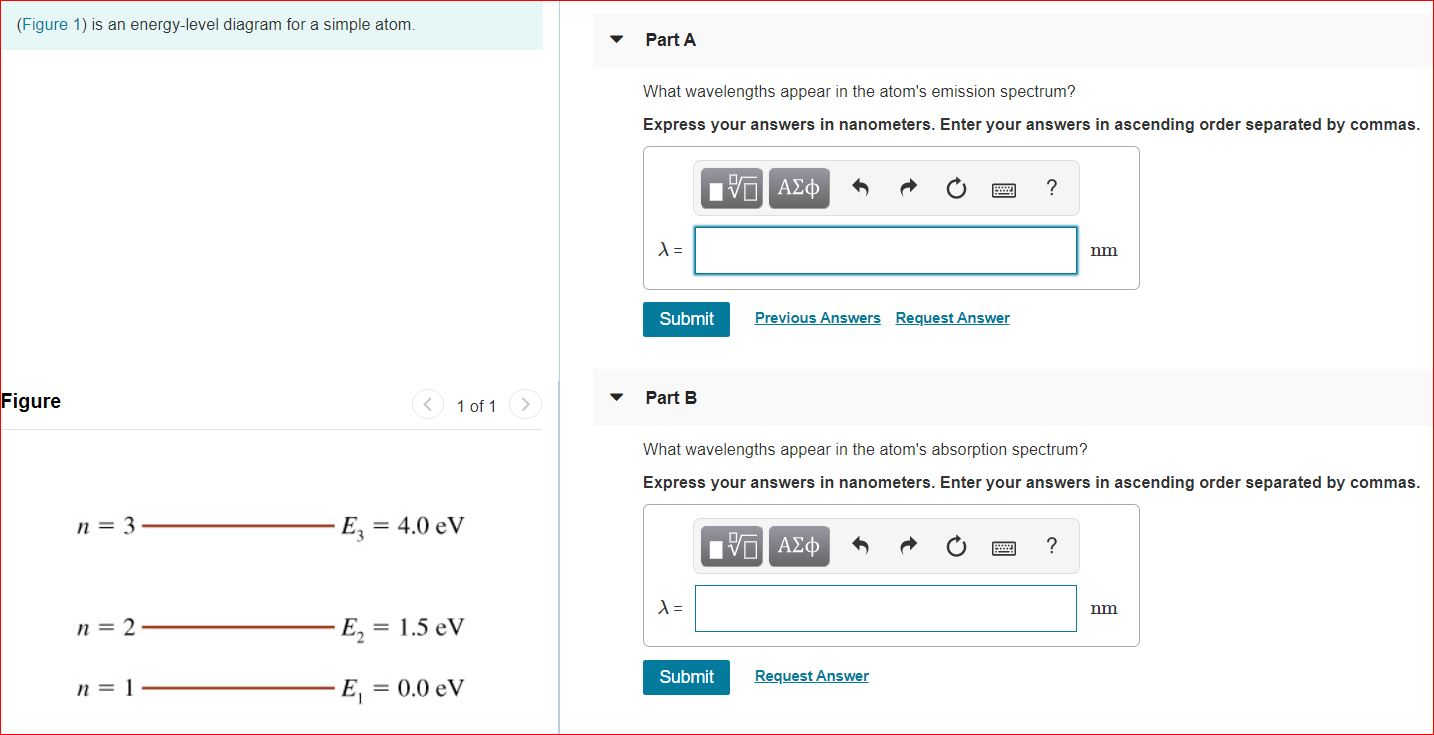
The figure (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? a.) 3 1 b.) 3 2 c.) 2 1 What wavelengths appear in the atom's absorption spectrum? d.) 1 2 e.) 1 3
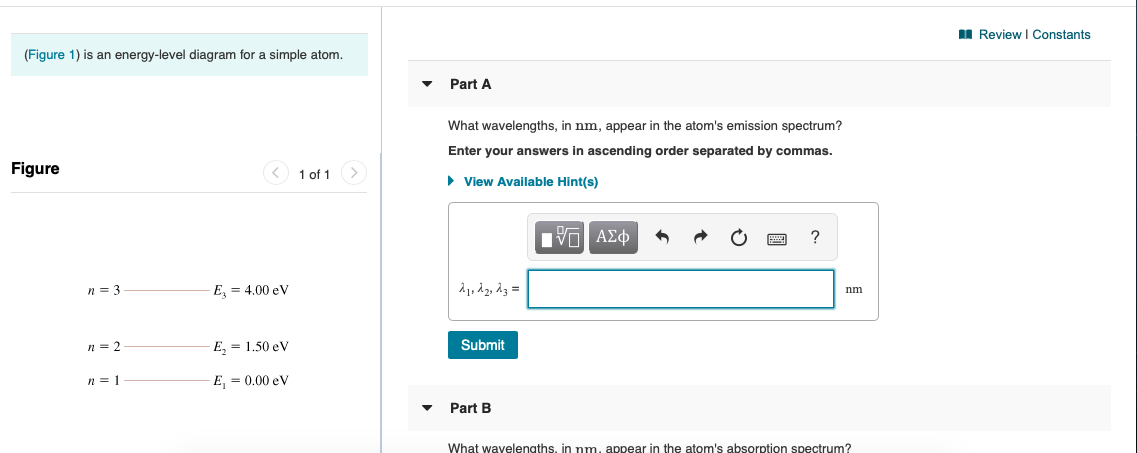
Question: (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas.
The figure is an energy-level diagram for a simple atom. (figure 1)
Solved Figure 1 Is An Energy Level Diagram For A Simple When an endothermic chemical reaction takes place heat is released into the environment. The figure is an energy level diagram for a simple atom figure 1. Overall is energy released or absorbed. Learn vocabulary terms and more with flashcards games and other study tools.
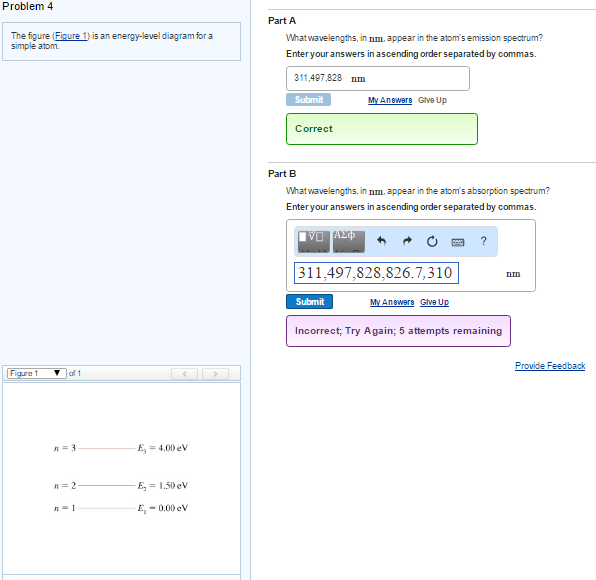
The figure (Figure 1) is an energy-level diagram for a simple atom. What wavelengths, in nm, appear in the atoms mission spectrum? Enter your answers in ascending order separated by commas. What wavelengths, in nm, appear in the atoms absorption spectrum? Enter your answers in ascending order separated by commas.
The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelength 3 to 1. #2: From wavelength 3 to 2. #3: From wavelength 2 to 1. What wavelengths appear in the atom's absorption spectrum?
The figure is an energy-level diagram for a simple atom. (figure 1).
Sep 8, 2020 — Figure 6.3.1: The Emission of Light by Hydrogen Atoms. ... Thus the energy levels of a hydrogen atom had to be quantized; in other words, ...
Get the detailed answer: The figure is an energy-level diagram for a simple atom. OneClass: The figure is an energy-level diagram for a simple atom. 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION →
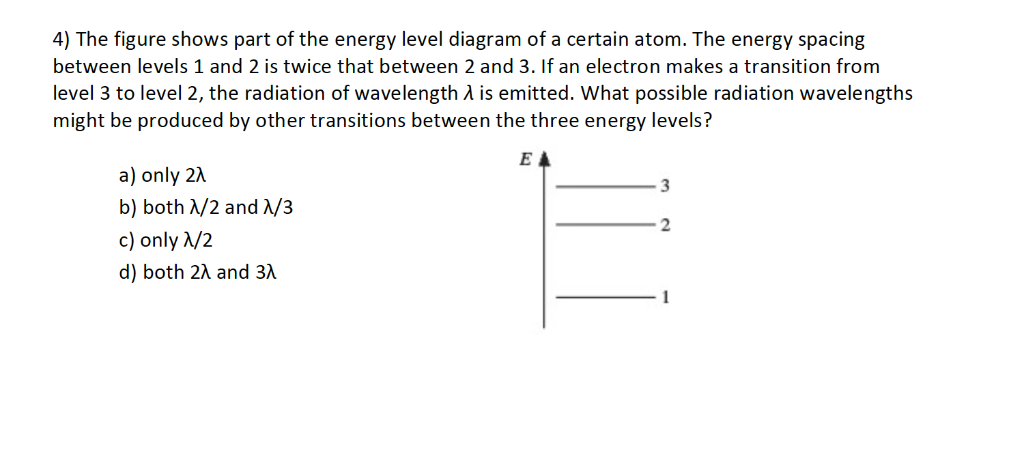
Figure 10.47 is the energy-level diagram for a particularly simple, fictitious element, Vernium (Vn). Indicate by the use of arrows all allowed transitions leading to the emission of. photons from this atom and order the frequencies of these photons from highest (largest) to lowest (smallest).
Fig. 12.-Calibrated electron impactYinduced fluorescence spectrum of H 2 using grating 2 at 100 eVelectron impact energy from 6500 to 7500 8. The stron- gest ...
Show transcribed image text (Figure 1) is an energy-level diagram for a simple atom. What wavelengths, in mm, appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths, in nm, appear in the atom's absorption spectrum? Enter your answers in ascending order separated by a comma (Figure 1) […]
The figure figure 1 is an energy level diagram for a simple atom. The figure is an energy level diagram for a quantum system. The figure figure 1 is an energy level diagram for a simple atom. You also observe that it takes 1750ev to ionize this atom. What wavelengths in nm appear in the atoms absorption spectrum.
The figure is an energy-level diagram for a simple atom. Subject: Physics Price: 2.85 Bought 3. Share With. The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelength 3 to 1 #2: From wavelength 3 to 2 #3: From wavelength 2 to 1. What wavelengths appear in the atom's ...
upwards with two protons (neutrons) in each available proton (neutron) energy level. Unlike Atomic Physics we do not even understand in principle what the ...
Figure 29.7 is an energy level diagram for 208Tl. What are the energies of the photons emitted for the six transitions shown?Figure 29.7 View Answer Show an energy level diagram for the MOs for He 2 and show how the electrons would be arranged in these MOs.
The figure figure 1 is an energy level diagram for a simple atom. What wavelengths appear in the atoms a emission spectrum and b absorption spectrum is broken down into a number of easy to follow steps and 24 words. From wavelength 3 to 1. The figure is an energy level diagram for a simple atom. What wavelengths appear in the atoms em. The ...
The figure below is an energy-level diagram for a simple atom, in which E_1 = 0.0 eV, E_2 = 17 eV, and E_3 = 3.3 eV. Calculate the wavelength(s) which appear(s) in the following two cases, in each of which you should submit all of the wavelength(s) in the single answer box, separated by spaces.
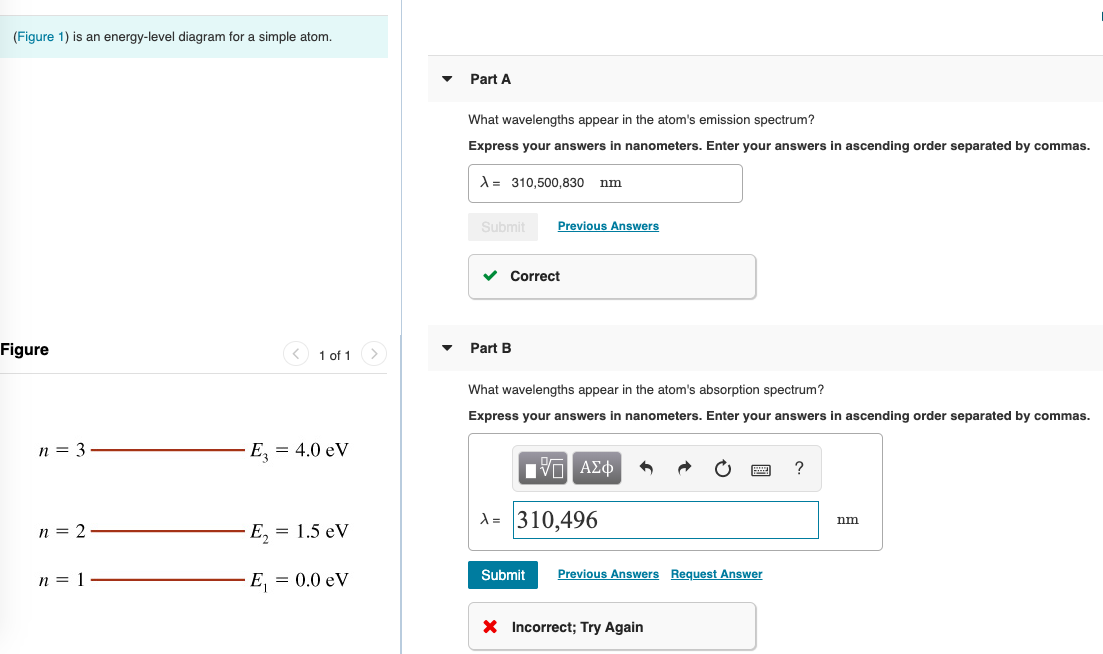
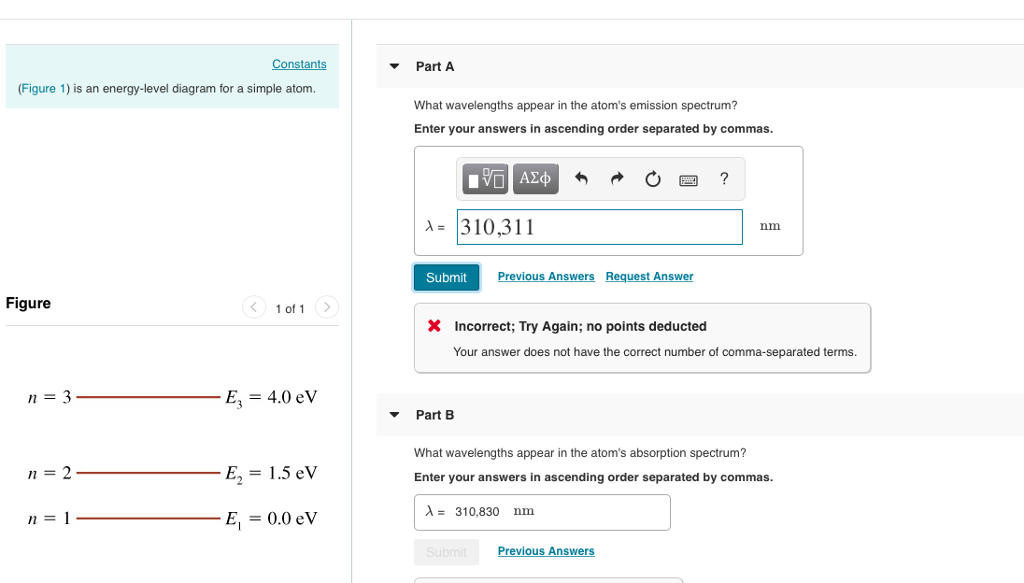
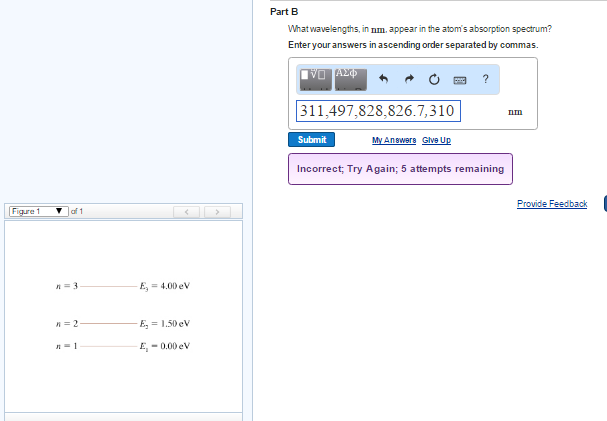
(Figure 1) is an energy-level diagram for a simple atom What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. lambda = 310, 500, 830 nm What wavelengths appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas.
The figure is an energy level diagram for a simple atom figure 1. What wavelengths appear in the atoms a emission spectrum and b absorption spectrum is broken down into a number of easy to follow steps and 24 words. A catalyst increases the rate of a reaction by increasing the net energy change of the reaction. What wavelengths in nm app.
in discussion were given the energies of a simple at all to be zero actual votes, four electoral votes and 60 actually cools. So this is the different energy levels, the energy level diagram. And you want to find the emission spectrum Where thinks yes, I see absorption spectrum. I sort of 40 bottom. The lowest energy just will be any quest, one every effort to and any costa tree to find What ...
The figure figure 1 is an energy level diagram for a simple atom. Biology8 chapter 6 pratice test. What wavelengths appear in the atoms em. The figure is an energy level diagram for a quantum system. I an electron with 20 ev of figure p298 kinetic energy collides with the in class 40 ev 830 nm 500 nm 310 nm 15 ev 830 nm 310 nm 00 ev 2 only 050 ...
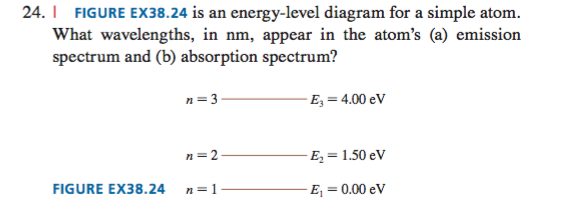
The figure is an energy level diagram for a simple atom figure 1. What wavelengths are ob. Draw the atoms energy level diagram. What wavelengths appear in the atoms a emission spectrum and b absorption spectrum is broken down into a number of easy to follow steps and 24 words. The figure figure 1 is an energy level diagram for a simple atom.
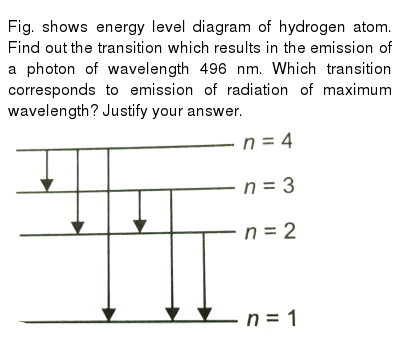
The figure indicates the energy level diagram for the origin of six spectral lines in the emission spectrum (e.g. line no. 5 arises from the transition from ...1 answer · Top answer: The spectral lines 4, 5 and 6 will not occur in the absorption spectrum. They are for the transitions from the higher excited state to lower excited state ...
The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelength 3 to 1 #2: From wavelength 3 to 2 #3: From wavelength 2 to 1 What wavelengths appear in the atom's absorption...
The figure figure 1 is an energy level diagram for a simple atom. Released because the energy level of the reactants is greater than that of the products. Consider the energy diagram for a chemical reaction in figure 6 3.
Get the detailed answer: The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelen Free unlimited access for 30 days, limited time only!
(Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order ...2 answers · 3 votes: Emission and absorption spectrum has same frequency (or wave length) Part A Emission spectrum ...
The figure figure 1 is an energy level diagram for a simple atom. What wavelengths appear in the atoms a emission spectrum and b absorption spectrum. Biology8 chapter 6 pratice test. I figure p298 is an energy level diagram for a simple atom. The labeled transitions a through e represent an electron moving between energy levels. What ...
Okay, so today we're going to talk about quantum transitions in atoms. Eso First of all, let's remember that the atoms can have two different types of transiti…
Show transcribed image text (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas. (Figure 1) is an energy-level diagram for […]
Quantum mechanics began with two deceptively simple formulas: ... Figure 1.14: Energy level diagram or “quantum ladder” for the one-dimensional infinite.






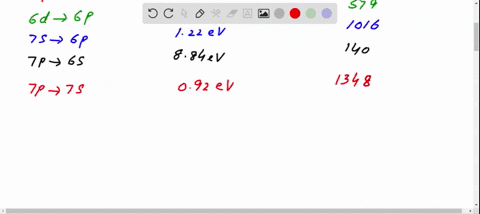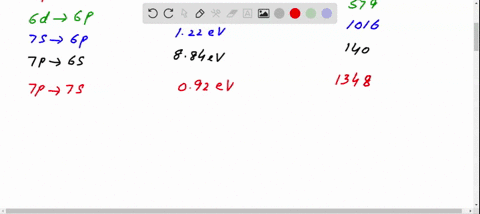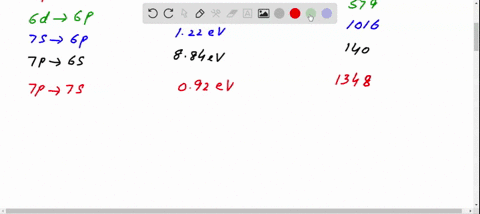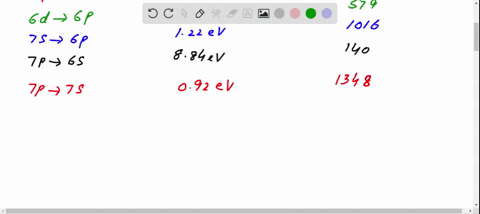











0 Response to "41 the figure is an energy-level diagram for a simple atom. (figure 1)"
Post a Comment