38 lewis dot diagram for chromium
Passivation, in physical chemistry and engineering, refers to coating a material so it becomes "passive," that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. How to draw the lewis dot structure for sbf4 The structure of monomeric cro3 has been calculated using density functional theory, and is predicted to be pyramidal (point group c3v) rather chromium trioxide decomposes above 197 °c, liberating oxygen and eventually giving cr2o3 4 cro3 + 3 rch2oh + 12 h+ → 3 rcooh + 4 cr3+ + 9 h2o.
The intestinal absorption of trivalent and hexavalent chromium (Cr) given orally (experiment I) or infused in the intestine (experiment II) was investigated in rats. The nonabsorbable form of chromium ((51)Cr2O3) and water-soluble and more absorbable Na2(51)CrO4 (the hexavalent form of Cr) were compared.Total retention of chromium given orally ranged around 15 percent of the dose, regardless ...
Lewis dot diagram for chromium
Chromium Electron Dot Diagram. Chromium Electron Dot Diagram. The electron configuration for chromium is NOT . The typical energy level diagram you see in text books showing the 4s below the 3d is ok up. The atomic number of Chromium is Z=24, therefore a Cr atom possesses 24 electrons. Cr:1s22s22p63s23p64s13d5. Lewis Structure of Chromate ion. There are four oxygen atoms and chromium atom in chromate ion. Also, there is -1 charges on two oxygen atoms. In the lewis structure of chromate ion contains two double bonds. Now, we are going to learn, how to draw the Lewis structure of CrO4 42- ion step by step. You will learn all steps and rules of Lewis ... Assisting students with assignments online. Get 24⁄7 customer support help when you place a homework help service order with us.
Lewis dot diagram for chromium. Answer: Chromium is element 24 and has electron structure [Ar]3d5 4s1, with 6 valence electrons. Chlorine is element 17 with electron configuration [Ne]3s2 3p5, with 7 valence electrons. In the +4 oxidation state, Chromium has lost 4 electrons, meaning that Chromium(IV) chloride will have the c... Bohr Model Lewis dot diagram. 1. Determine the element's symbol 2. Determine the number of electrons 3. Determine number of valence electrons 4. Now draw your ... Chromium 51.996 Molybdenum 95.94 w Tu ngsten 183.85 106 [266] Periodic Table of the Nickel 58.693 Palladium 106.42 pt Platinum 195.08 110 [269] Elements IVA Chromium Because Chromium has 24 protons/electrons on a Bohr Diagram it will have 24 dots. On a Lewis Dot it will have 6 dots because of Chromium's group number. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. Element: Bohr Diagram; Group Number (PT) # of Valance Electrons; Lewis Dot Structure; Calcium. Carbon. Hydrogen. Helium. Oxygen. Fluorine. Neon. Sodium. Aluminum. Determining …
Draw the Bohr Diagram for Chromium (Cr). Cr with 2e on 1st energy level, 8e on 2nd, 14e on 3rd. 300. ... Draw a Lewis Dot Structure using the following electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 1. In with 3 dots. 500. Order these based on increasing wavelength: red, gamma, microwaves, blue, and UV. ... The Lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. The Lewis dot structure for chromium is Cr with two dots on top and... (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol ... Lewis dot structures made easy - Electron structures of the chromate anion CrO4-2, lewis electron dot structure of chromate anion cro4-2, σ bonds in chromate anion cro4-2, π bonds in chromate anion cro4-2, calculate the number of electrons in π bonds, resonance structures in chromate anion cro4-2, octet rule in chromate anion cro4-2, Lewis dot structures for chromate anion cro4-2, resonance ...
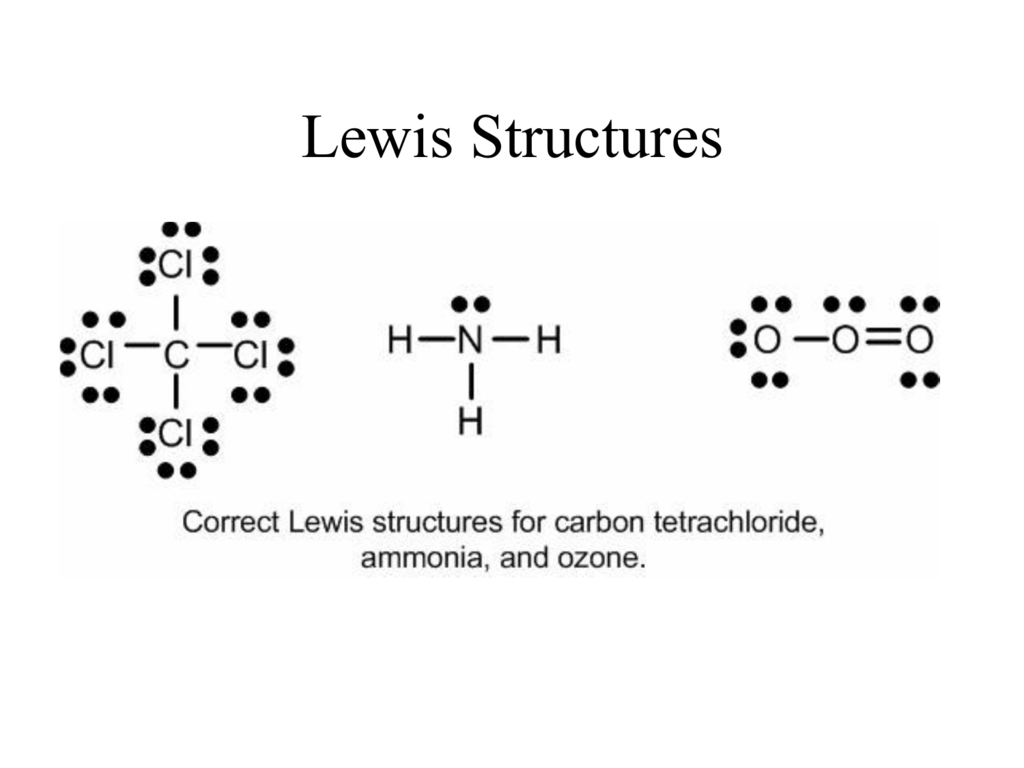
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Jul 5, 2019 · Uploaded by Wayne Breslyn 21/10/2021 · Each dot represents a gene. (G) t-SNE projection of chromatin accessibility and gene expression profiles from A20 and K562 cells in the SNuBar-ARC experiments. (H) Venn diagram showing number of cells detected by one or both assays in SNuBar-ARC. Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ...
Apr 25, 2021 — Chromium is element 24 and has electron structure [Ar]3d5 4s1, with 6 valence electrons. Chlorine is element 17 with electron configuration ...
Nov 4, 2021 — Chromium Electron Dot Diagram Skater chart please help !!!! Am I so stupid? 3 Well, today we are studying dot graph in cl and I was ...
Jun 22, 2017 · 1 answerChromium is element 24 and has electron structure [Ar]3d5 4s1, with 6 valence electrons. · Chlorine is element 17 with electron configuration [Ne]3s2 3p5, with 7 ...What is the electron configuration of Cr? - Quora7 answersDec 4, 2016How many valence electrons does Chromium have ...1 answerMar 16, 2018How is Gallium's Lewis dot symbol different from its ...3 answersNov 16, 2020More results from www.quora.com
Lewis Dot Diagram For Tellurium. Tellurium (Te) has an atomic mass of Find out about its Electron Configuration, [Kr] 5s2 4d10 5p4. 1s2 2s2 2p6 3s2 Lewis Dot Diagram of Tellurium (Te). Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Exercises. 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. 2.
To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
Hydrogen is a colorless, odorless gas. It is easily ignited. Once ignited it burns with a pale blue, almost invisible flame. The vapors are lighter than air. It is flammable over a wide range of vapor/air concentrations.
The Lewis dot structure for chromium is. Drawing and Predicting Lewis Structures Learning Goal. Show the formal charges of all atoms in the correct structure. Now let us try Lewis dot structure of Sulfide ion S 2-Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons instead of 16.
If you are asked to write the Lewis Structure of Manganese (Mn) you'll first need to find the number of valance electrons for Iron. One of the challenges in ...
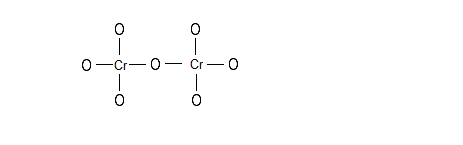
PubMed. Chromium (III) oxide (or chromia) is an inorganic compound with the formula ChromiumCr 2 OxygenO 3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite .
What is represented by the dots in a Lewis electron-dot diagram of an atom of an element in period 2 of the periodic table? ... A. chromium (II) phosphate B. chromium (III) phosphate C. chromium (II) phosphide D. chromium (II) phosphide. B. Sets found in the same folder. chemistry final exam review.
A {eq}\displaystyle 0.1246 g {/eq} sample of a compound of chromium and chlorine was dissolved in water. All of the chloride ions were captured by silver ions in the form of {eq}\displaystyle AgCl.
15/07/2021 · Draw Lewis diagrams for an atom of each of the following elements: Li, N, F, Na. Solution. We find from the periodic table inside the front cover that Li has an atomic number of 3. It thus contains three electrons, one more than the noble gas He. This means that the outermost, or valence, shell contains only one electron, and the Lewis diagram is
To find the number of valence electrons for Chromium (Cr) we need to look at its electron configuration. This is necessary because Cr is a transition metal ...
Dot| Lewis structures of chromium trioxide CrO3 |Simple method for writing Lewis structures | Lewis configuration, vita min, draw for, lewis structures and the octet rule, chromium and sugar and fat metabolism, chromium and action of insulin, dietary supplements and vitamins, chemical formula of CrO3, single bonds in CrO3 resonance, double bonds in CrO3 resonance, resonance structures of CrO3 ...
Carbon dioxide is a one-carbon compound with formula CO2 in which the carbon is attached to each oxygen atom by a double bond.A colourless, odourless gas under normal conditions, it is produced during respiration by all animals, fungi and microorganisms that depend directly or indirectly on living or decaying plants for food.
Because Chromium has 24 protons/electrons on a Bohr Diagram it will have 24 dots. On a Lewis Dot it will have 6 dots because of Chromium's group number. Comprehensive information for the element Chromium - Cr is provided by this page including scores of properties, element names in many languages, most known nuclides and .
When drawing an electron dot diagram, the nucleus is represented by the atomic symbol, which will be in the center of the diagram. GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. The Lewis Structure for Li is Li with one dot to the right of the element. Dot • one dot represents one valence electron (found on odd-electron particles).
How do you draw electron dot diagrams? How to Draw a Lewis Dot Structure. Determine the total number of valence electrons to be depicted in the Lewis diagram. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine how many electrons must be added to central element.

Caption reads, "[John Lewis speaking at a meeting of American Society of Newspaper Editors, Statler Hilton Hotel, Washington, D.C.] / [MST]." Original black and white negative by Marion S. Trikosko. Taken April 16th, 1964, Washington D.C, United States (@libraryofcongress). Colorized by Jordan J. Lloyd. Library of Congress Prints and Photographs Division Washington, D.C. 20540 https://www.loc.gov/item/2003688130/
We've gathered our favorite ideas for Chromium Lewis Dot Structure, Explore our list of popular images of Chromium Lewis Dot Structure and Download Photos Collection with high resolution
Chromium Bohr Diagram and Lewis Dot Diagram; ... Chromium. Some of the everyday uses for Chromium are things like: forks, magnetic tape, leather, wood, stainless steel, knives, paint,and glasswear cleaner. Powered by Create your own unique website with customizable templates.
How to draw a Lewis Dot Structure. Click card to see definition 👆. Tap card to see definition 👆. Draw dots for electron at NSEW. draw electron singly first, before pairing. count the columns of the periodic table from left to right and skip the transition metals. 1st column = 1 valence electron, for example, H.
Comprehensive information for the element Chromium - Cr is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
29/09/2021 · Electron Dot Diagram of CO 2. The valence shell of an oxygen atom includes six electrons. Four of the valence electrons are in lone pairs, meaning that in order to achieve an octet configuration, the oxygen atom must engage in two single bonds or one double bond. Because an O 2 molecule has just two oxygen atoms, the atoms form a double bond, resulting …
Assisting students with assignments online. Get 24⁄7 customer support help when you place a homework help service order with us.
Lewis Structure of Chromate ion. There are four oxygen atoms and chromium atom in chromate ion. Also, there is -1 charges on two oxygen atoms. In the lewis structure of chromate ion contains two double bonds. Now, we are going to learn, how to draw the Lewis structure of CrO4 42- ion step by step. You will learn all steps and rules of Lewis ...
Chromium Electron Dot Diagram. Chromium Electron Dot Diagram. The electron configuration for chromium is NOT . The typical energy level diagram you see in text books showing the 4s below the 3d is ok up. The atomic number of Chromium is Z=24, therefore a Cr atom possesses 24 electrons. Cr:1s22s22p63s23p64s13d5.










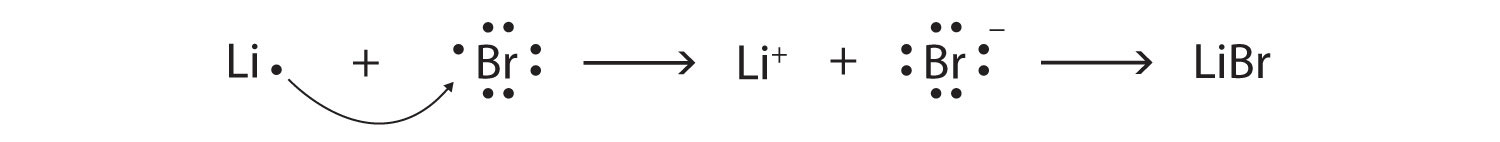






0 Response to "38 lewis dot diagram for chromium"
Post a Comment