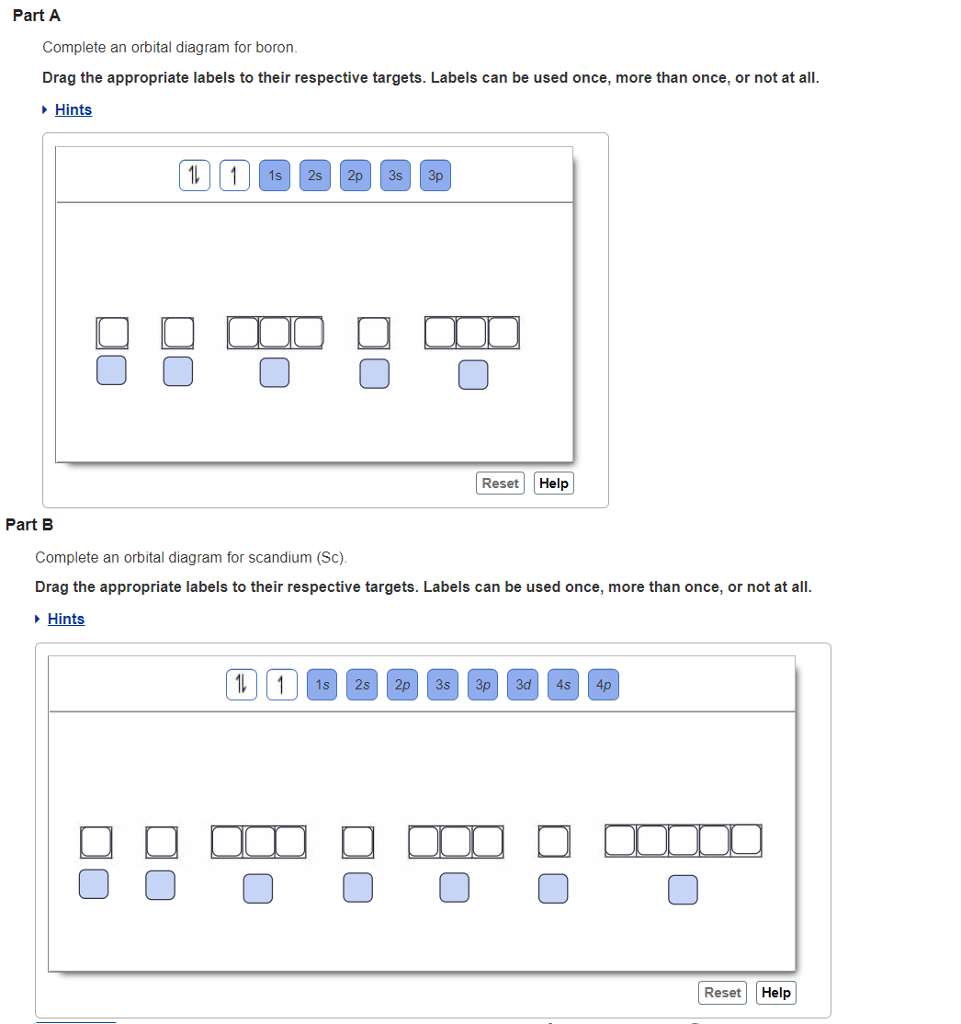
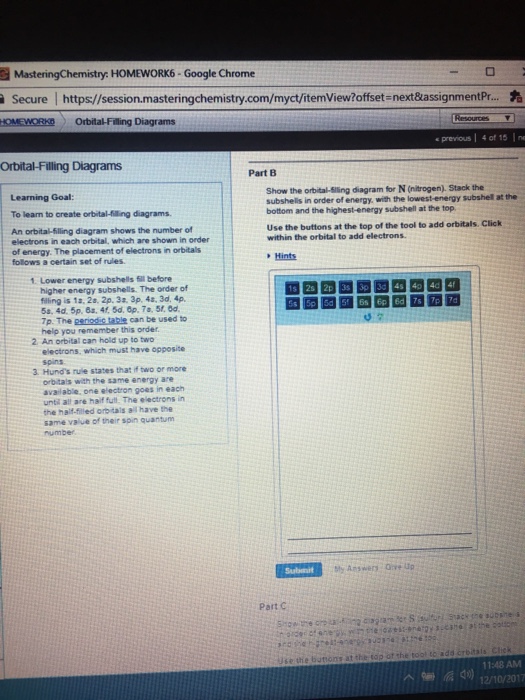
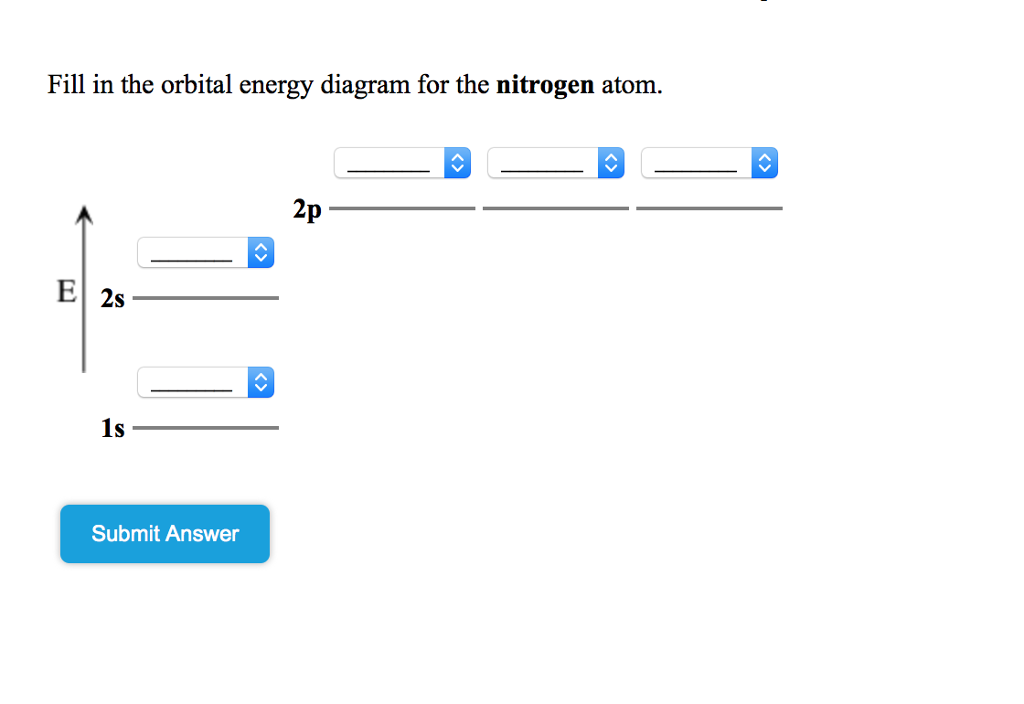
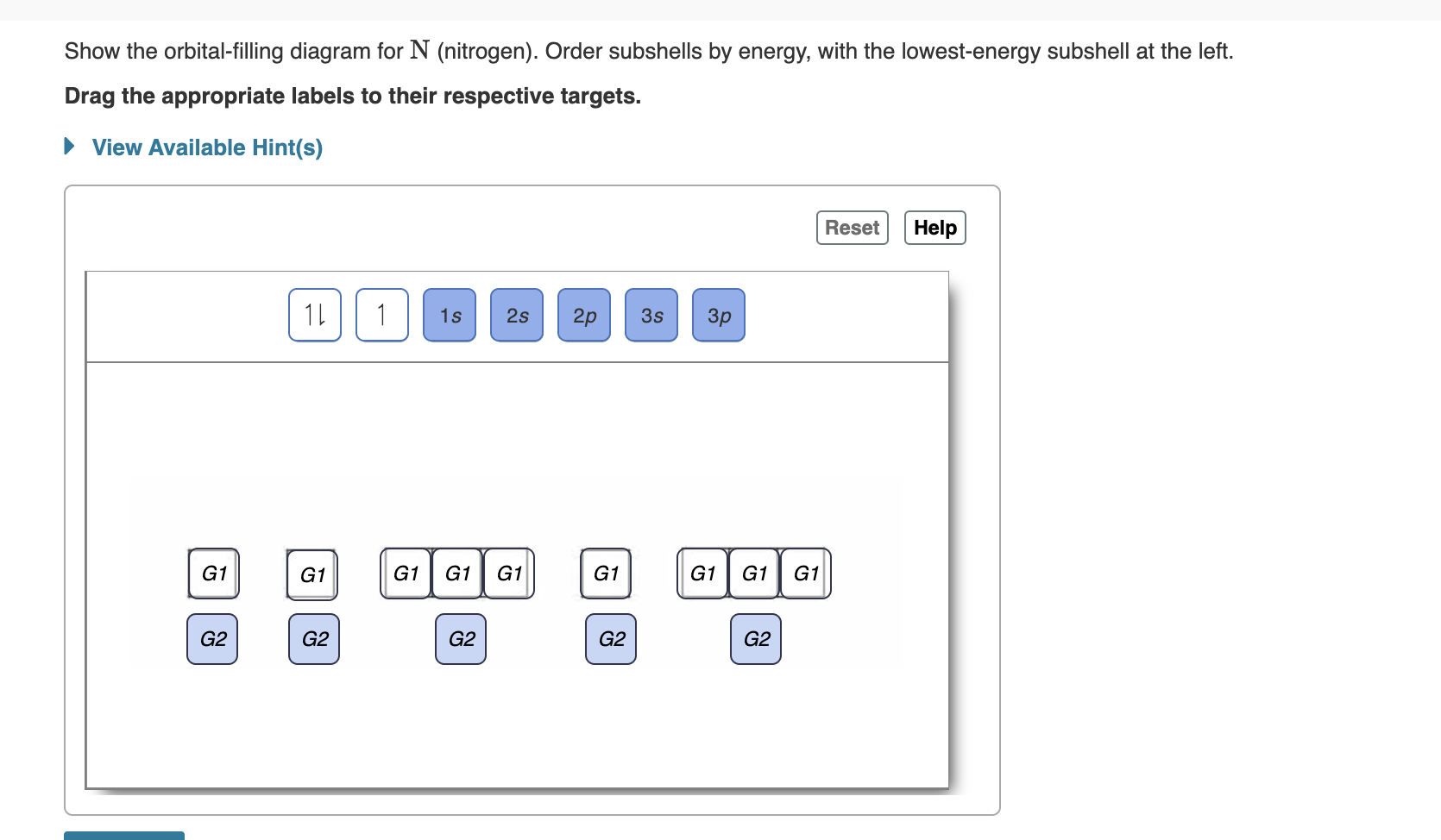
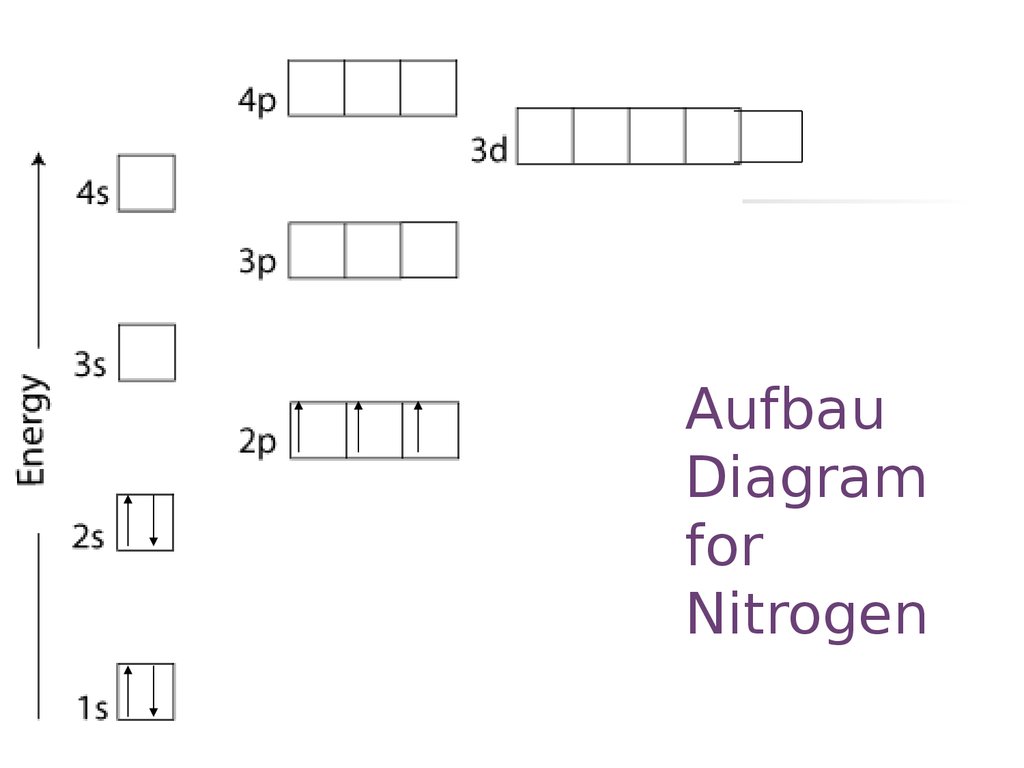
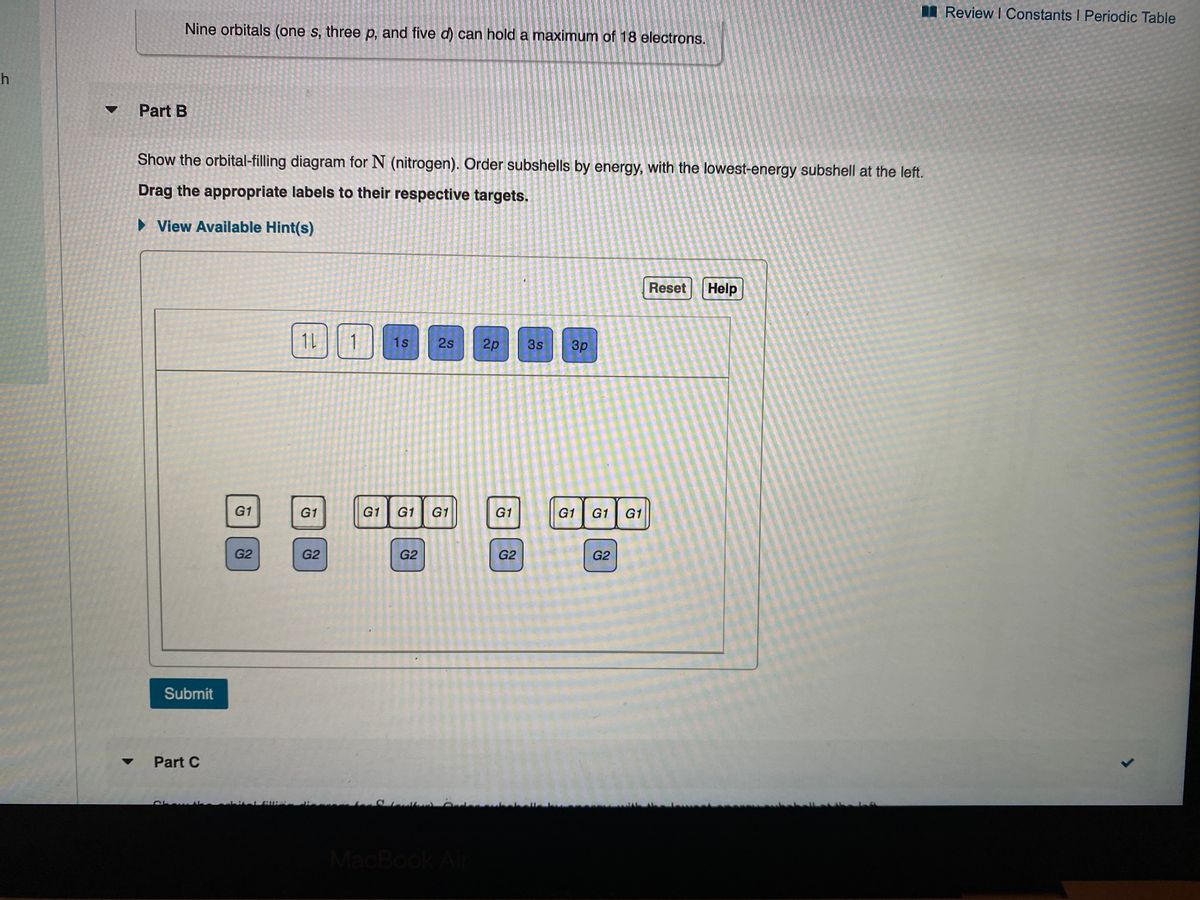
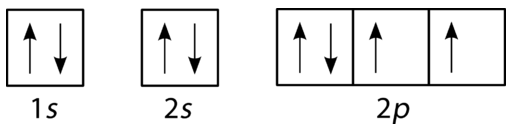
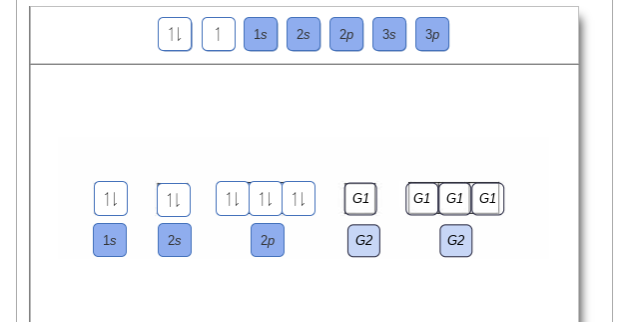
38 show the orbital filling diagram for n nitrogen
of oxygen is 1s 2 2s 2 … An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. As another example, oxygen has 8 electrons. The electron configuration can be written as 1s 2 2s 2 2p 4. The orbital diagram is Mar 18, 2019 · Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add % (8). Question: Orbital Diagrams Draw an orbital diagram for boron.
analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal Electron Configurations and Orbital Box Diagrams Transfer of electron(s) is involved in the formation of an electrovalent bond.

Show the orbital filling diagram for n nitrogen
Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons not shown), giving a total bond order of three. Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons. The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell. Show the orbital-filling diagram for N (nitrogen)%(8). The outer-most electrons are the only ones included in the orbital filling diagram and the electron dot diagram because the outer-most electrons are the only ones that need to be used in chemical reactions and bonding, so the other electrons are insignificant in these diagrams.
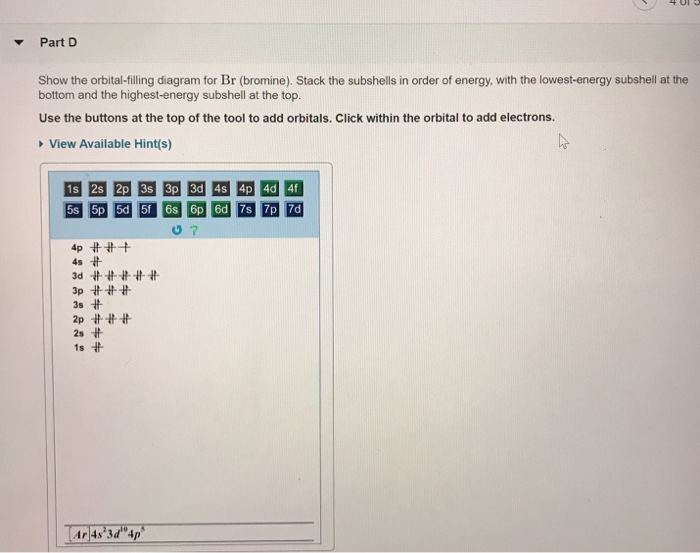
Show the orbital filling diagram for n nitrogen. Draw An Orbital Diagram For Boron. Mar 18, 2019 · Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add %(8). Question: Orbital Diagrams Draw an orbital diagram for boron. orbital diagram is drawn as follows: the first 2 electrons will pair up in the 1s orbital; the next 2 electrons will pair up in the 2s orbital. That leaves 4 electrons, which must be placed in the 2p orbitals. According to Hund's Rule, all orbitals will be singly occupied before any 03/10/2012 · Practice Problems (Chapter 8): Electron ... #2*3=6# electron in each #p# orbital #2*5=10# electron in each #d# orbital, and so on so forth. Electron orbitals fill according to the Aufbau (Build-up) Principle. That is, each added electrons fill orbitals of lower energies before filling those of higher energies, so as to minimize the electrostatic potential energy within the atom. Properties of V N in Pure AlN. Nitrogen vacancies, the main source of n-type nature of AlN, are naturally present in aluminum nitride crystals. To explore the nitrogen vacancy effects in pure AlN, a comparative view of TDOS and PDOS plots of Al-3 s, Al-3p, N-2 s, and N-2p for pure AlN and V N:AlN is shown in Fig. 2.Fermi energy for AlN and V N:AlN is set to be at zero.
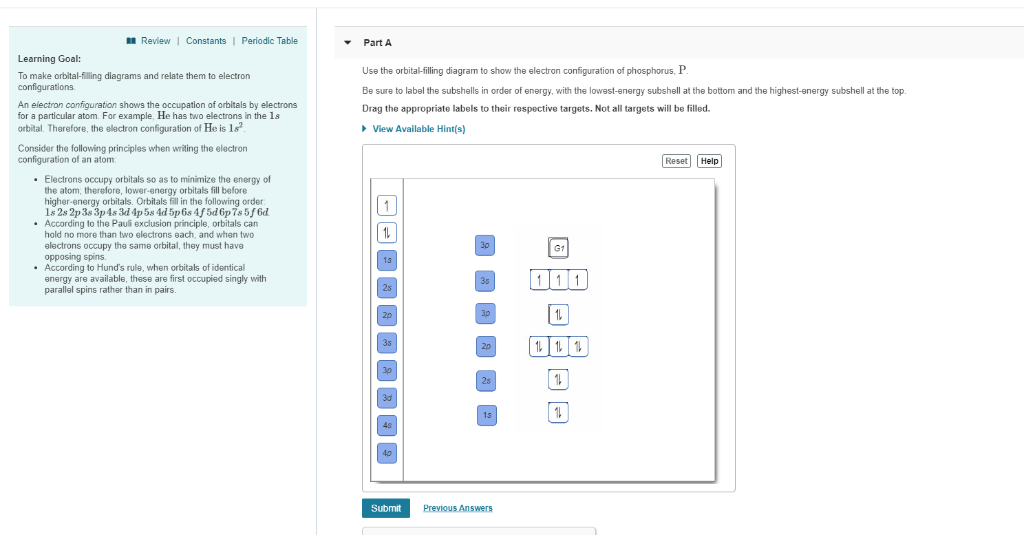
electron configuration and orbital diagram of: (a) Na + (b) P 3- (c) Al 2+ (d) Fe 2+ (e) Sm 3+ Solution First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want The CH2O is a tetra atomic molecule where the bond angles for the hydrogen-carbon-hydrogen (H-C-H) and hydrogen-carbon-oxygen (H-C-O) are 116° and 122° and the structure is bent shaped. Moreover, the Valence Shell Electron Pair Repulsion (VSEPR) theory, says the molecular geometry of a molecule is trigonal planar if the bond angle is 120° or ... slight chance of the electron being a considerable distance away from the nucleus. Thus, the attempts to show probabilities as a fuzzy cloud are limited to where the electron may be 90% of the time. Sample Questions - Chapter 5 In this equation, h is Planck's constant and E i and E f are the initial and final orbital energies, respectively. Since there is 1 electron in the box labeled 1s, we say the H electron configuration in orbital notation is 1s 1. The orbital notation can also be interpreted as quantum numbers, where the principal quantum number n is the energy level (1 before the s), the azimuthal quantum number corresponds to the letter s, and the spin quantum number is +1/2.
For Nitrogen, its atomic number is 7, so after 2 electrons occupy 's' orbital, the rest 5 are in the outer orbital so the valence number of electrons is 5. Now to find the total number of valence electrons we will add up the valence electrons of all three atoms: =1+4+5 = 10 valence electrons. Show The Orbital Filling Diagram For N Nitrogen Atkinsjewelry . In each box the spin of an electron is noted by using arrows up arrows mean 12 spin and down arrows mean 12 spin. Blank orbital filling diagram. Oct 26 2016 3 min read. An orbital can hold a maximum of two electrons. Paired Unpaired. First write a column of s orbitals from 1 to 8. related to: molecular orbital diagram bn. www.a3bs.com. Shop Our Molecular Orbitals - World Leader In Anatomy Models. Explore Our Excellent Selection Of Molecular Orbitals & More. Shop & Save Today! Show the orbital-filling diagram for N (nitrogen)%(8). The outer-most electrons are the only ones included in the orbital filling diagram and the electron dot diagram because the outer-most electrons are the only ones that need to be used in chemical reactions and bonding, so the other electrons are insignificant in these diagrams.
The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.
Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons not shown), giving a total bond order of three. Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons.

Mowing the lawn and weeds of an old historic home in Smithfield Utah. Located at TK Secure Storage in Logan & Brigham City, Utah. www.tksecurestoragelogan.com www.tksecurestoragebc.com

















0 Response to "38 show the orbital filling diagram for n nitrogen"
Post a Comment