39 ch4 electron dot diagram
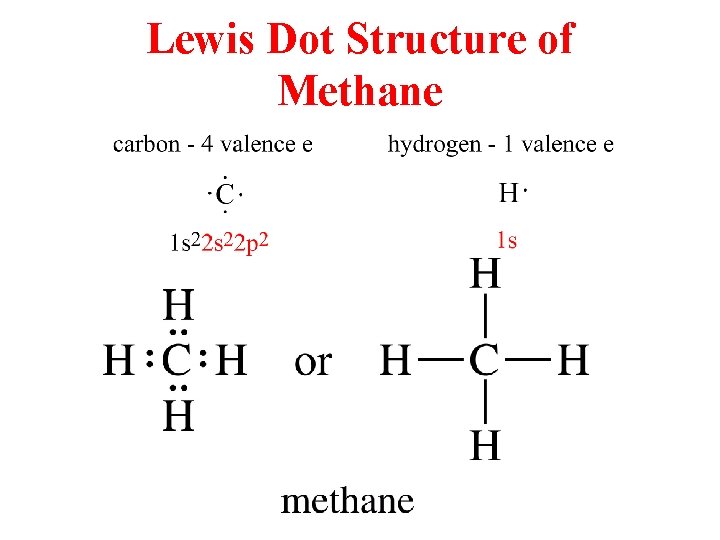
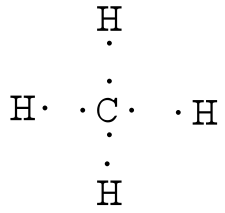
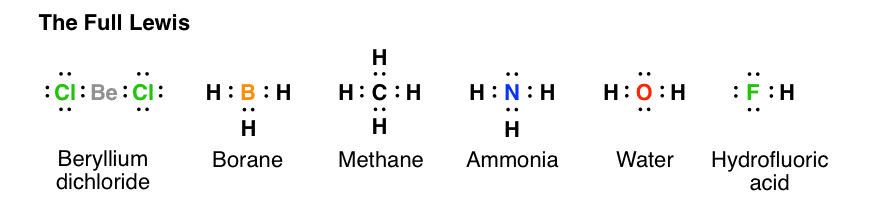
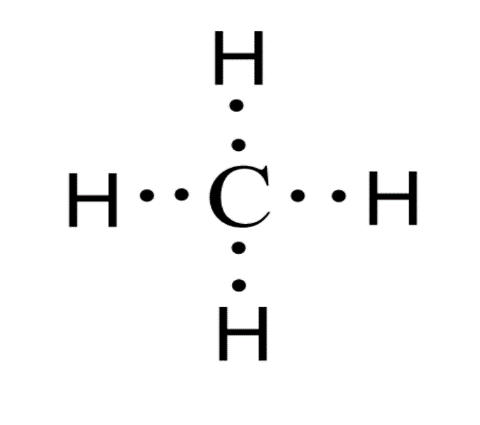
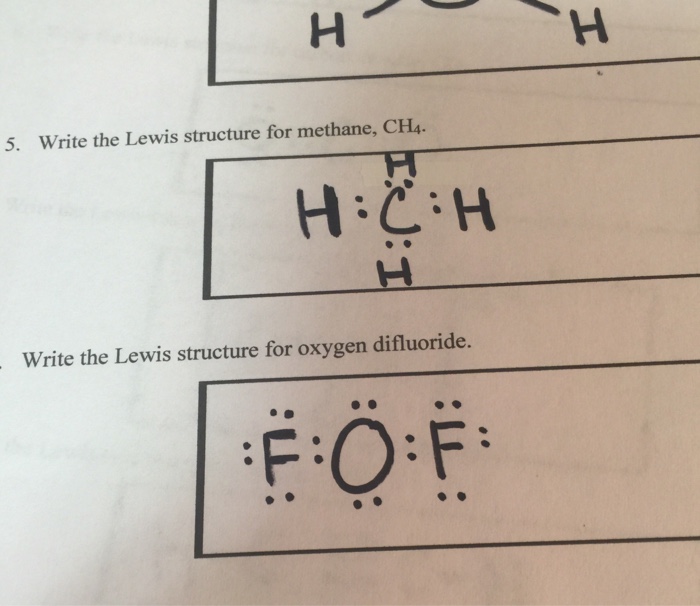
For CH4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH4 (named methane) requires only single bonds. It's one of the easier ...28 Oct 2016 · Uploaded by Wayne Breslyn The chemical formula of methane is CH4. It is a gas that exists abundantly in nature. Now, let's know its structure: Lewis dot structure is a representation of ...1 answer · Top answer: Hint: To answer this question we should be aware of the chemical formula of methane, Lewis dot structure and the type of bond formed. This information will ...
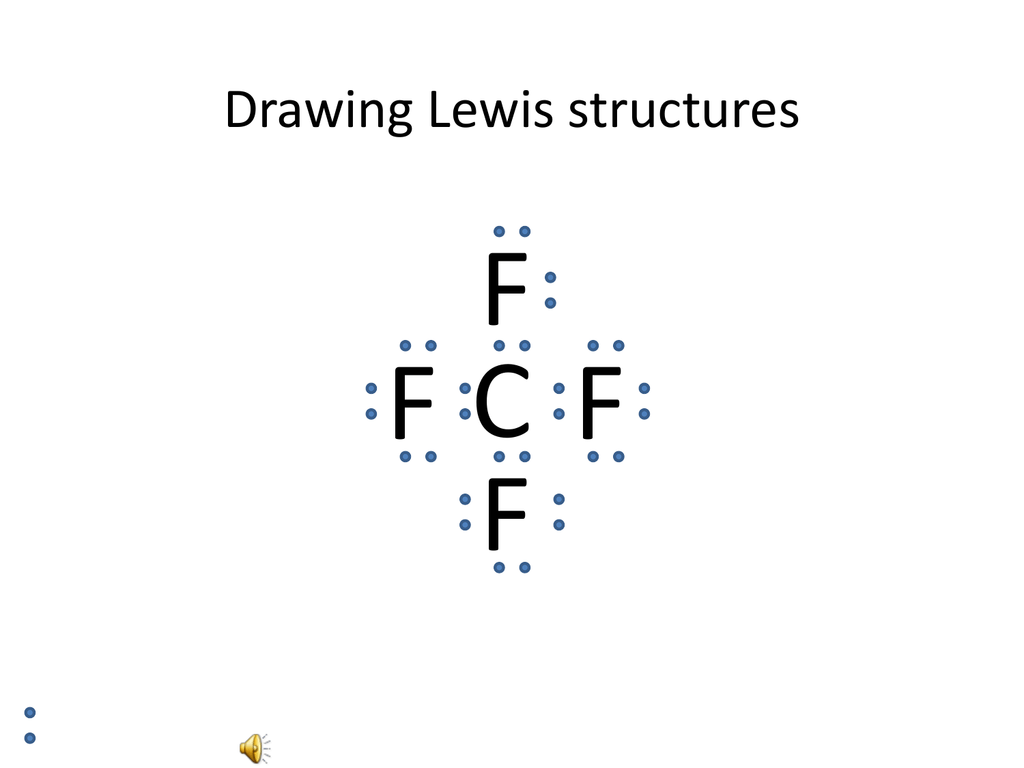
This is the final Lewis Dot Diagram. The pairs of dots represent shared electrons and you can see that the Carbon possesses 8 and the Hydrogens possess 2 ...1 answer · 1 vote: I will explain this with pictures, and some captions. This is just the five atoms in ...

Ch4 electron dot diagram
Solution · In CH4, the central atom is a carbon. In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its ...1 answer · Top answer: In CH4 , the central atom is a carbon.In electron dot structure we represent the valence electron of the element.Thus, Carbon has 4 electrons in its ... The total valence electron available for the Nitrogen trichloride lewis structure is 26. The hybridization of NCl3 is Sp³. Nitrogen trichloride is slightly polar in nature. The molecular geometry of NCl3 is trigonal pyramidal and its electron geometry is tetrahedral. NCl3 lewis dot structure contains 1 lone pair and 3 bonded pairs. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to ...17 Dec 2020 · Uploaded by Wayne Breslyn
Ch4 electron dot diagram. Follow some steps for drawing the lewis dot structure of CH2O. 1. Count total valence electron in CH2O. As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. Valence electrons are the outermost electron of an atom that can participate in the bond formation either by donating or accepting. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to ...17 Dec 2020 · Uploaded by Wayne Breslyn The total valence electron available for the Nitrogen trichloride lewis structure is 26. The hybridization of NCl3 is Sp³. Nitrogen trichloride is slightly polar in nature. The molecular geometry of NCl3 is trigonal pyramidal and its electron geometry is tetrahedral. NCl3 lewis dot structure contains 1 lone pair and 3 bonded pairs. Solution · In CH4, the central atom is a carbon. In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its ...1 answer · Top answer: In CH4 , the central atom is a carbon.In electron dot structure we represent the valence electron of the element.Thus, Carbon has 4 electrons in its ...
























0 Response to "39 ch4 electron dot diagram"
Post a Comment