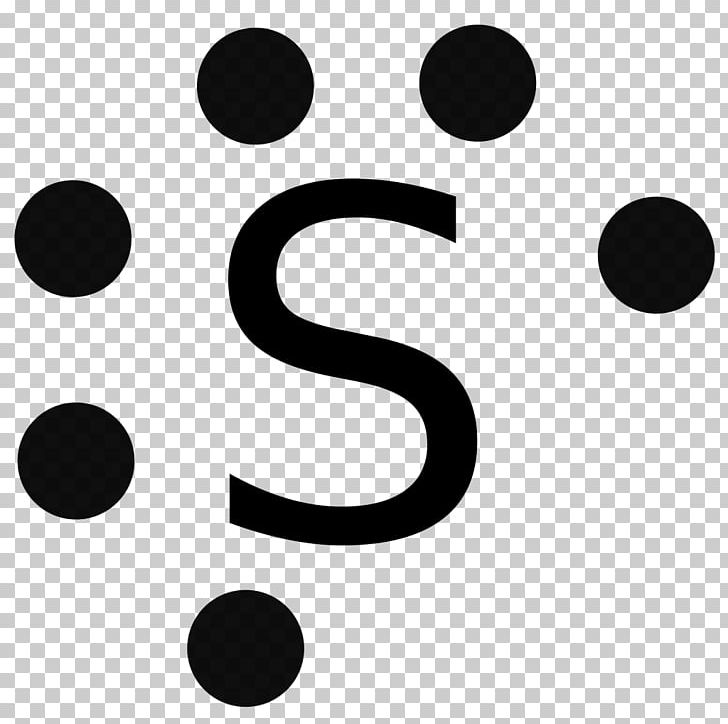
40 electron dot diagram for sulfur
also sulphur, c. 1300, from Anglo-French sulfere, Old French soufre "sulfur, fire and brimstone, hellfire" (13c.), later also sulphur, from Late Latin sulfur, from Latin sulphur, probably from a root meaning "to burn." Ousted native brimstone and cognate Old English swefl, German schwefel, Swedish swafel, Dutch zwavel. The spelling with -ph- is standard in Britain, but its suggestion of a Greek origin is misleading. 14+ Electron Dot Structure Of H2S. The electron dot structure are as follows The single electrons from each of the two hydrogen atoms are shared when the atoms come together to form a hydrogen molecule (h2). Draw the electron dot structures for (a) ethanoic acid. (b … from eguruup.com.
The Lewis dot structure for Sulfur is an S with 6 dots which stand for its six valence electrons. What is the Lewis structure for magnesium oxide? Magnesium oxide is an ionic compound.
Electron dot diagram for sulfur
and cross diagram. ions ClF3 BrF3 IF3 T-shaped molecules linear shaped molecules ClF2- the valence shell Therefore, when applying VSEPR to molecules shape and bond Acute (short-term) inhalation exposure to phosphine may cause headaches, dizziness, fatigue, drowsiness, burning substernal pain, nausea, vomiting, cough, labored breathing, chest tightness, pulmonary irritation, pulmonary edema ... SCl2 is used to synthesize other sulfur compounds like SOCl2, S4N4, S3H2, etc. In this article, we will understand the concepts of Lewis dot structure, hybridization, and polarity. We will also learn to determine the geometry and shape of a covalent compound using VSEPR theory. SCl2 Lewis Structure Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below.
Electron dot diagram for sulfur. "pattern consisting of dots of uniform size and arrangement," especially on fabric, 1851 (polka-spot and polka-dotted are used in 1849), when they were in fashion, from polka (n.) + dot (n.). Named for the dance, for no reason except its popularity, which led to many contemporary products and fashions taking the name (polka hat, polka-jacket, etc.). They had a revival in fashion c. 1873. Related: Polka-dots. Sulfur will share 6 electrons with one electron of each Fluorine atom. Here is a pictorial representation of the same. A bond formation is shown by a single straight line and lone pairs of electrons are shown by two dots for each pair. Now when we know that how many bonds are getting formed, let us see how many lone pairs will be made. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. Sf4O Lewis Structure. Covalent bonds and molecular structure. Also, there is a lone pair on sulfur atom and three lone pairs on each fluorine atom. A ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. In sf4 lewis structure, each fluorine atom has made single bonds with center sulfur.
Sulfur has six valence electrons in its outer shell. Fluorine has seven valence electrons. Total number of valence electrons for SF2 - 6 + 7*2 ( as there are two atoms of Fluorine, we will multiply the number by 2) = 6 + 14 = 20 valence electrons So, Sulphur Difluoride has a total of 20 valence electrons. SF2 Lewis Structure 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). Hydrogen electron configuration is 1s 1.Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.. The first element of the periodic table is hydrogen and its position at the beginning of the periodic table. coined 1891 by Irish physicist George J. Stoney (1826-1911) from electric + -on, as in ion (q.v.). Electron microscope (1932) translates German Elektronenmikroskop.
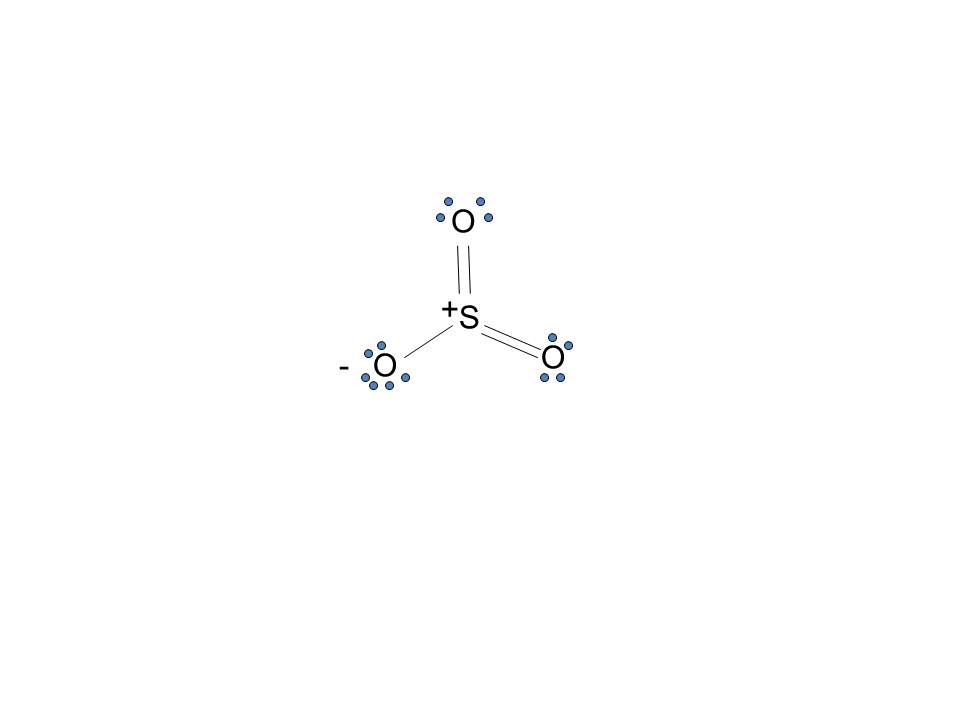
What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagram s. Magnesium reacts with sulfur to produce sulfide a in. That is, magnesium is a cat ion element. Magnesium donates the electron of the last shell to for m bonds and turns into ... Sulfur has six electrons, while oxygen (O2) has four electrons at the external level, among which one electron is used for every bond. So, in five pairs, there are 10 electrons, among which two different double bonds use two pairs together and are shaped as a single entity. Formula: Valence electrons - Dots - No. of lines = '?' If we talk about Sulfur, there will be 6 - 0 - 3 = +3. Here in this case, for oxygen, we have 6 (oxygen) - 6 (dots) - 1 (bonds) = -1. So, -1 from the other 2 oxygen because all are the same! The sulfur can handle 12 electrons per atom. Sulfur is the central atom and contains 2 lone pairs whereas both hydrogen is connected to the central atom with the help of a single bond. Lewis’s structure of SH2 is really helpful to determine its electron geometry, molecular shape, number of shared pair, and lone pair electrons. Follow these steps to draw the lewis dot structure for H2S
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
A sulfur atom on the outer level has six electrons, and four are used by Oxygen for each bond. That leaves a total number of ten electrons in five pairs. One lone pair is left while two double bonds form as a unit, making the Bent or V shape. Molar Mass You can calculate the molar mass by using the usual formula n=N/NA (then N=n*NA).
1740, "mark with a dot or dots," from dot (n.). Sense of "mark or diversify with small, detached objects" is by 1818. Sense of "put a dot over (the letter i)" is by 1833. Related: Dotted; dotting. Dotted line is by 1690s.
There are two S=O. bonds and two S-O bonds in sulfate ion lewis structure. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom. Is SO4 2 an acid or base? Only in a very acidic environment would HSO4- act as a base.
Jul 25, 2020 — 1: A valid electron dot structure for sulfur. The presence of unpaired electrons within an atom is inherently destabilizing. Therefore, if two ...
1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
What is the electron dot structure for CH4? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
The electron configuration for O 2 – is 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 3. This leaves 1 unpaired electron and gives a bond order of 1.5. (b) Use the same MO diagram as in (a), giving an electron configuration for O 2 + of: 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 1. This leaves 1 unpaired electron and gives a bond order of 2.5.
Lewis dot structure will have 4 paired dots around Sulfur atom.For atoms and monoatomic ions, step one is sufficient to get the correct Lewis structure.
The Lewis Structure of SCl2, sulfur dichloride, has a sulfur atom (which brings six valance electrons) bonded two chlorine atoms (which each bring seven valence electrons). By drawing the Lewis Structure accurately, we can determine the shape and hybridization of SCl2 as well.
hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic...
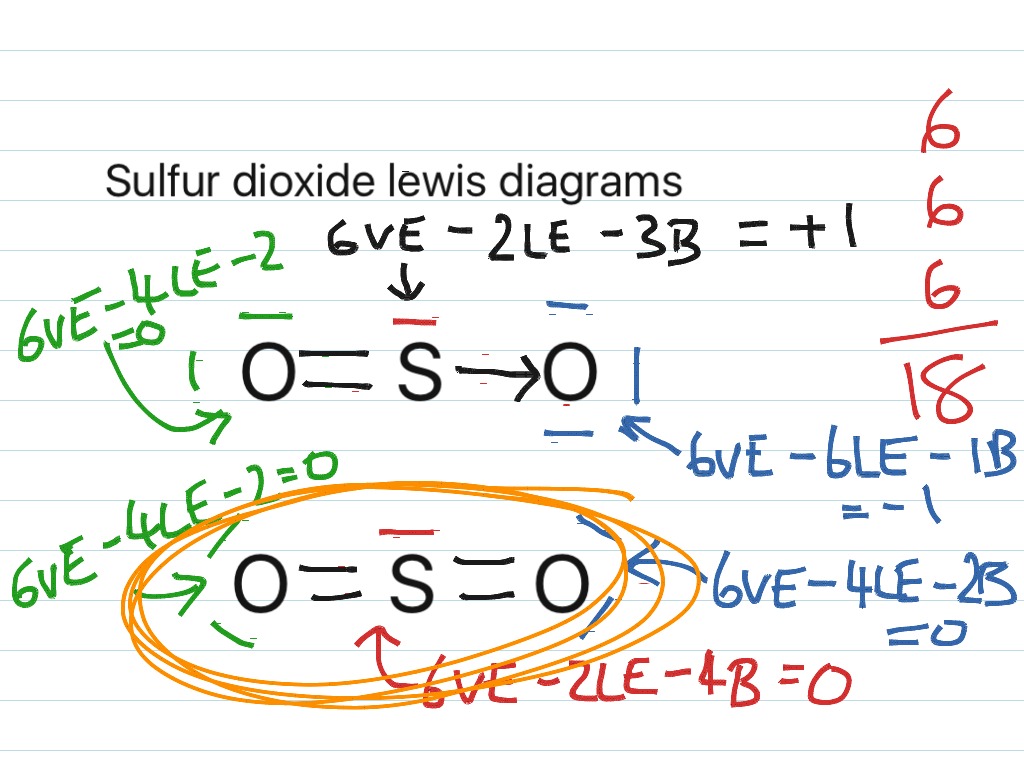
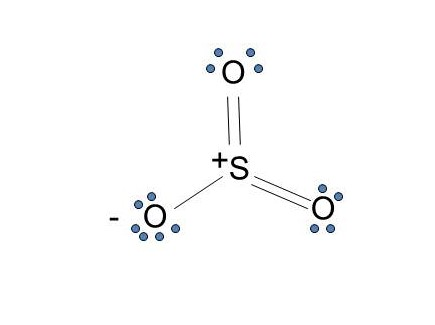
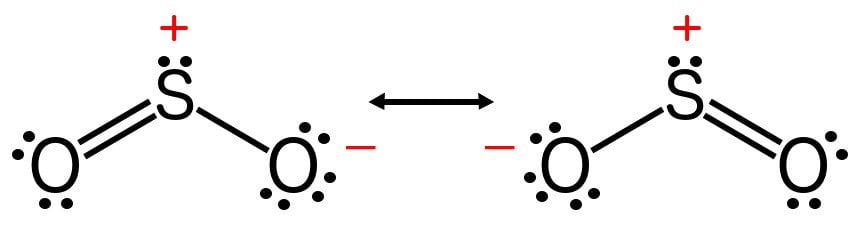
The Lewis structure most closely resembling reality consists of two resonance structures. Sulfur dioxide SO 2 Lewis Structure Hybridization. This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. We start with a valid Lewis. Three stable resonance structures can.
Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom. For atoms and monoatomic ions, step one is sufficient to get the correct Lewis structure.
prefix usually meaning "away, opposite, completely," from Old English for-, indicating loss or destruction, but in other cases completion, and used as well with intensive or pejorative force, from Proto-Germanic *fur "before, in" (source also of Old Norse for-, Swedish för-, Dutch ver-, Old High German fir-, German ver-); from PIE *pr-, from root *per- (1) "forward," hence "in front of, before, toward, near, against." In verbs the prefix denotes (a) intensive or completive action or process, or (b) action that miscarries, turns out for the worse, results in failure, or produces adverse or opposite results. In many verbs the prefix exhibits both meanings, and the verbs frequently have secondary and figurative meanings or are synonymous with the simplex. [Middle English Compendium] Probably originally in Germanic with a sense of "forward, forth," but it spun out complex sense developments in the historical languages. Disused as a word-forming element in Modern English. Ultimately from the same root as fore (adv
Old English for "before, in the sight of, in the presence of; as far as; during, before; on account of, for the sake of; in place of, instead of," from Proto-Germanic *fur "before; in" (source also of Old Saxon furi "before," Old Frisian for, Middle Dutch vore, Dutch voor "for, before;" German für "for;" Danish for "for," før "before;" Gothic faur "for," faura "before"), from PIE root *per- (1) "forward," hence "in front of, before," etc. From late Old English as "in favor of." For and fore differentiated gradually in Middle English. For alone as a conjunction, "because, since, for the reason that; in order that" is from late Old English, probably a shortening of common Old English phrases such as for þon þy "therefore," literally "for the (reason) that."
Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen.
"point or minute spot on a surface," Old English dott, once, "speck, head of a boil," perhaps related to Norwegian dot "lump, small knot," Dutch dot "knot, small bunch, wisp," Old High German tutta "nipple;" a word of uncertain etymology. Known from a single source c. 1000; the word reappeared with modern meaning "mark" c. 1530; not common until 18c. Perhaps this is a different word imitative of "the mark of a mere touch with the pen" (Wedgwood). In music, the meaning "point indicating a note is to be lengthened by half" is by 1806. Morse telegraph sense is from 1838. On the dot "punctual" is 1909, in reference to a clock dial face. Dot-matrix in printing and screen display is attested by 1975.
In the outer level, Sulphur has six electrons, and the Oxygen has four of them among which one electron is used for each bond. So total number of ten electrons in five pairs. To make bonds, four pairs are needed, so one pair remains alone. The two double bonds use two pairs each and form as a single unit.
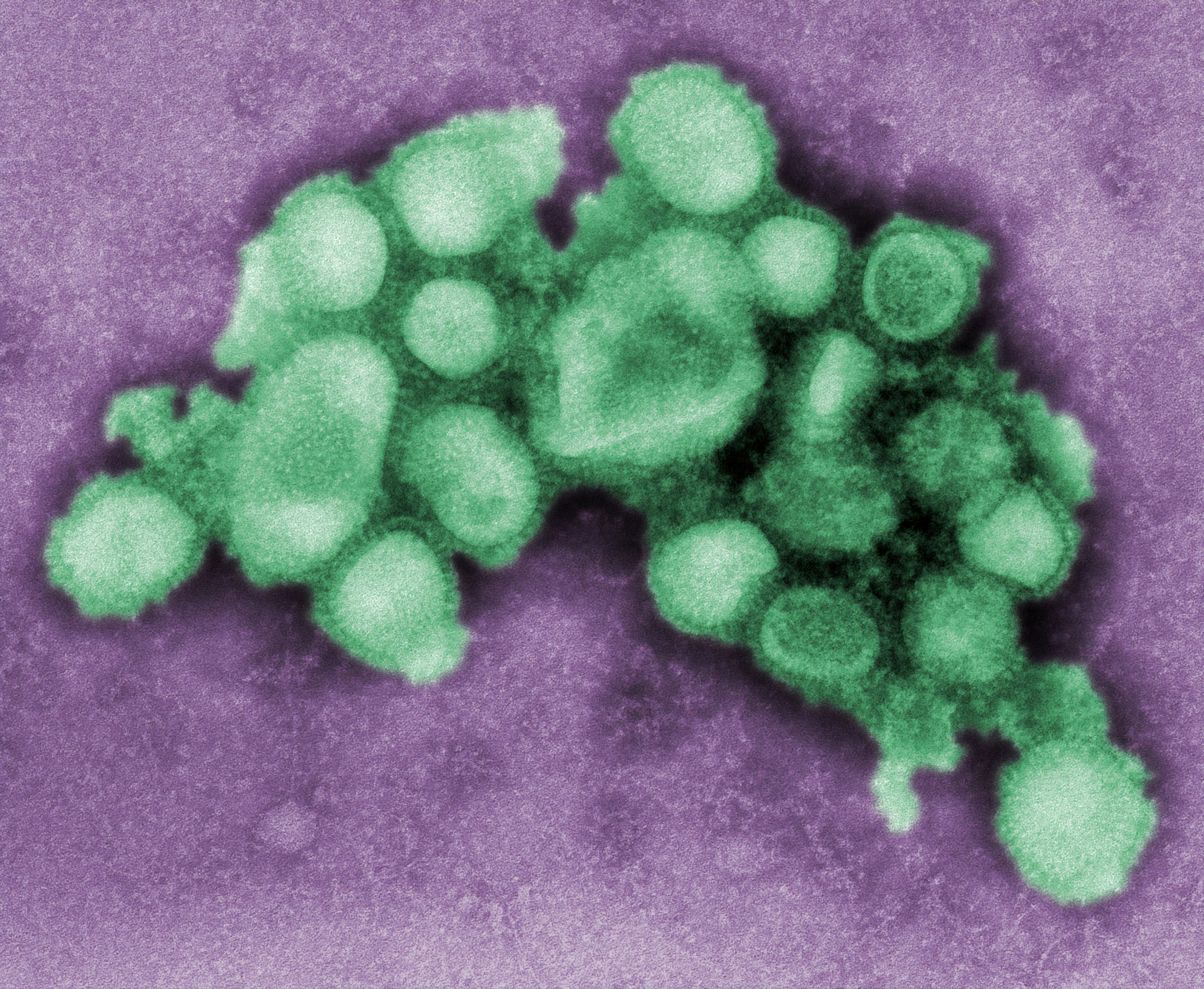
This digitally-colorized, negative-stained transmission electron microscopic (TEM) image depicted some of the ultrastructural morphology of the A/CA/4/09 Swine Flu virus.
Jul 23, 2018 — Sulfur is in group 16/VIA, so its atoms have six valence electrons. The Lewis dot symbol for an element represents the valence electrons as ...1 answer · Refer to the explanation. Explanation: Sulfur is in group 16/VIA, so its atoms have six valence electrons. The Lewis dot symbol for an element represents ...
1550s, "be taken or regarded as," also "be in favor of," from go (v.) + for (adv.). Meaning "attack, assail" is from 1880. Go for broke is from 1951, American English colloquial.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
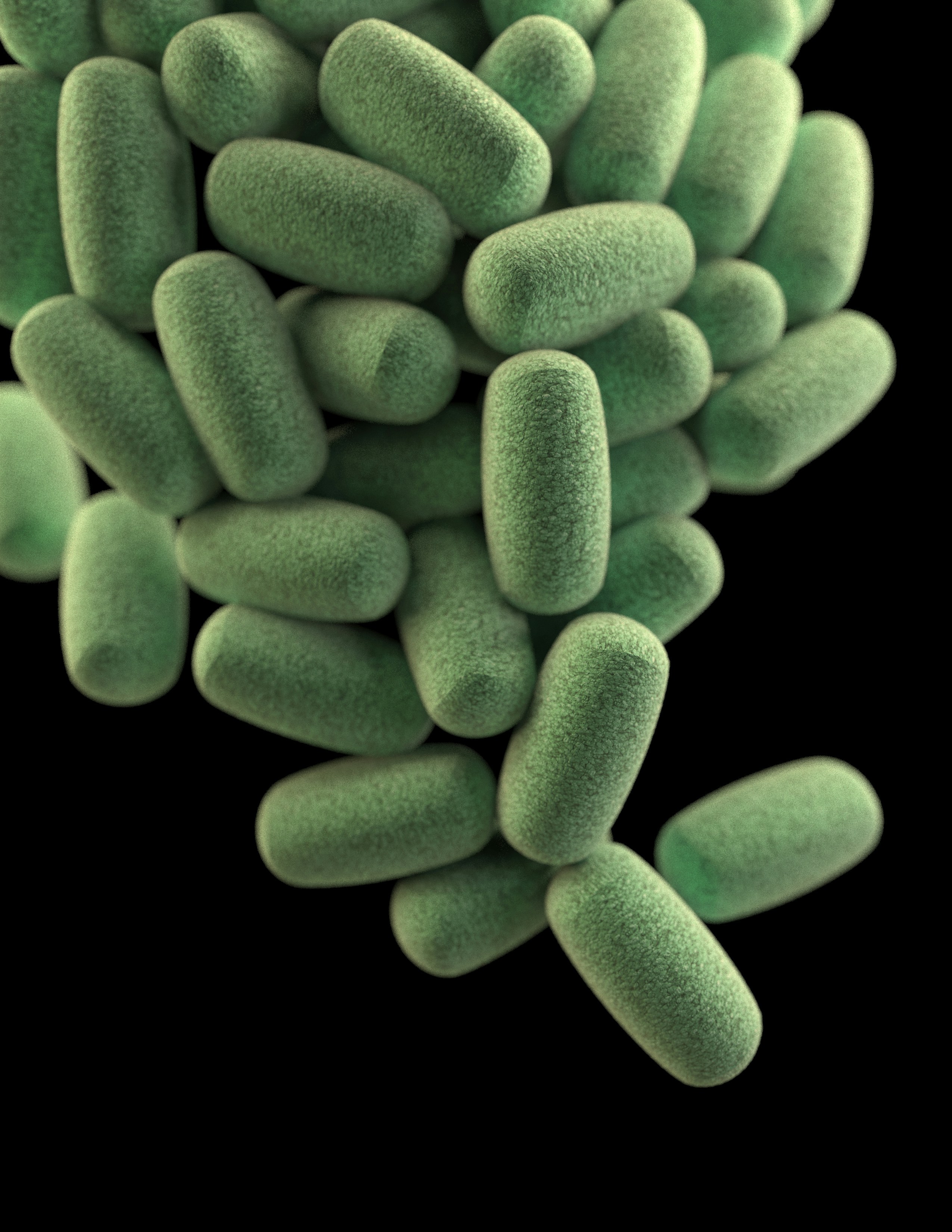
This illustration depicts a three-dimensional (3D), computer-generated image of a cluster of barrel-shaped, Clostridium perfringens bacteria. The artistic recreation was based upon scanning electron microscopic (SEM) imagery. See PHIL 21914, for another view of these microbes.
It is figured out by the variety of electrons an atom brought - number of lone pair of electron - half the number of electrons in shortcut formation. So, for oxygen, the is 6-6-1 = -1 And for sulfur, it is 6-0-3 = +3.
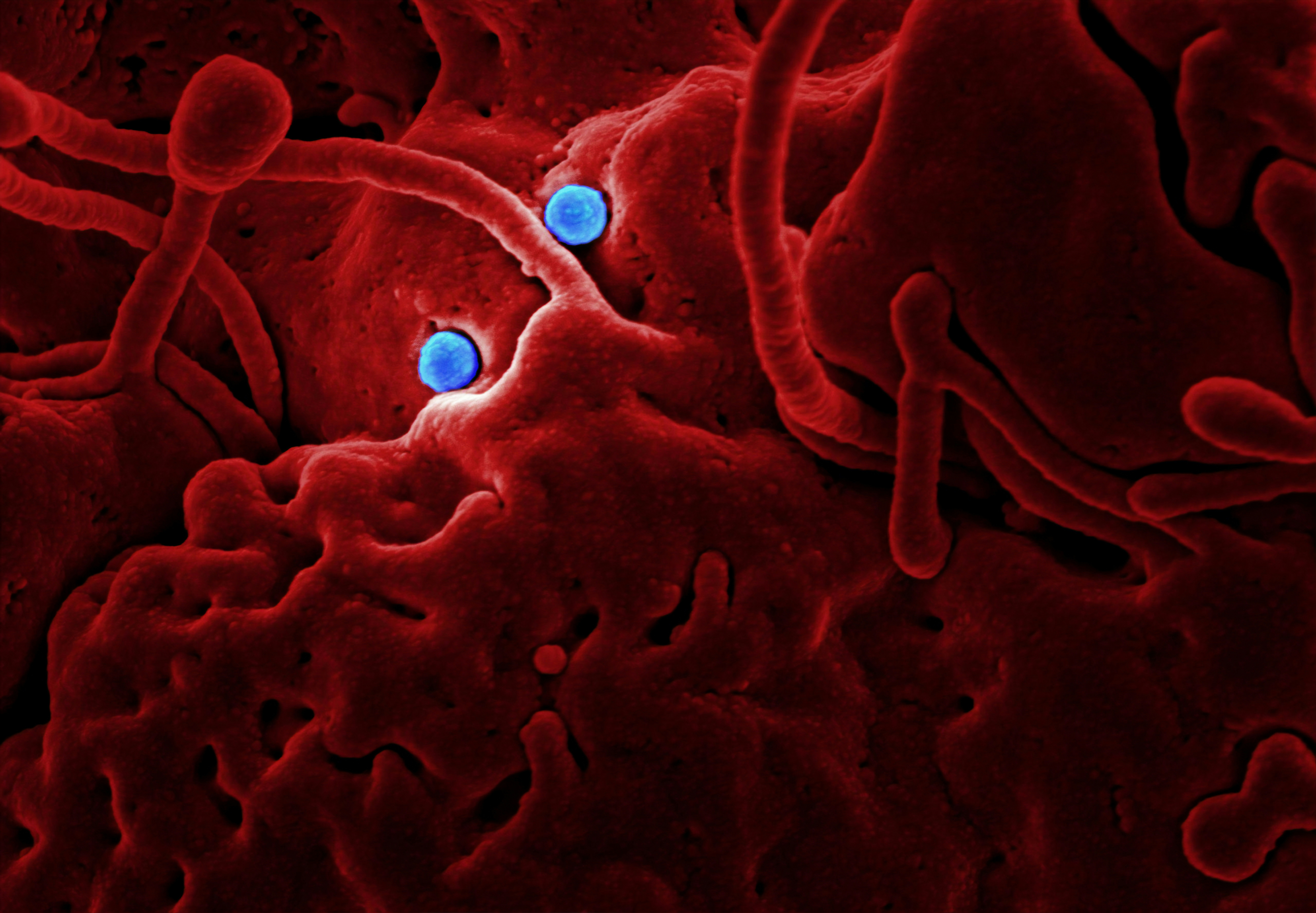
Produced by the National Institute of Allergy and Infectious Diseases (NIAID), in collaboration with Colorado State University, this highly magnified, digitally colorized scanning electron microscopic (SEM) image, reveals ultrastructural details at the site of interaction of two spherical shaped, Middle East respiratory syndrome coronavirus (MERS-CoV) viral particles, colorized blue, that were on the surface of a camel epithelial cell, colorized red.
Sulfur being the less electronegative atom than chlorine atom is placed at the center in lewis’s diagram and chlorine is spaced evenly around it. There is two lone pair present on the central atom and this central atom attached to two bonded pair in SCl2 lewis structure. Steps to draw electron dot structure or lewis structure of SCl2
An energy level diagram is shown below. Since helium has two electrons in the 1s orbital then it is considered a closed orbital. The next atom lithium has three electrons. This third electron must go into the next lowest energy orbital, the 2s. The electron configuration can also be summarized by writing the symbol for the occupied subshell and
Lewis electron dot diagram for HSiN. Drawing the Lewis Structure for PO 3 3-For the PO3 3- Lewis structure we first count the valence electrons for the PO3 3- molecule using the periodic table. The Sulfur bromide SBr6 OC-6-11- molecule contains a total of 6 bonds There are 6 non-H bonds. Lewis electron dot diagram for SiF4.
*Continuing* I wrap the six road flares, now spray-painted brick-red and stickered with the appropriate manufacturer's labels, with black electrician’s tape into a hexagonal cross-section, closest-fit bundle. I have a black plastic project box that contains a battery for ‘long-lasting power’ or so the manufacturer claims. An Arduino board that I programmed the other night that runs the wee little speaker and set of blinking LEDs I had mounted on the box. From the box sprout a pair of tightly c...
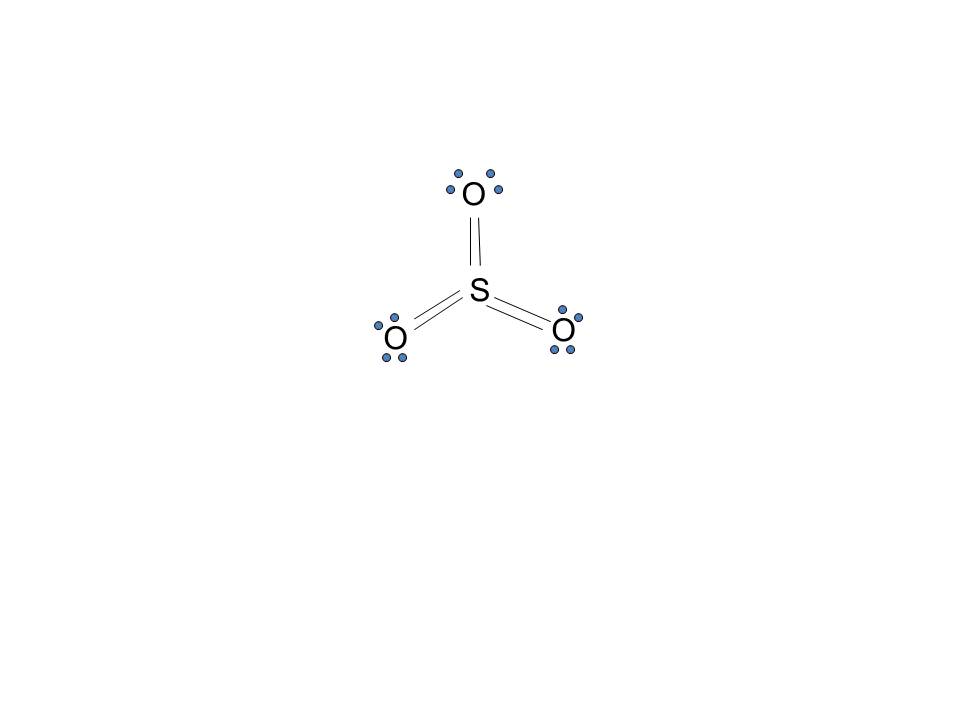
In SO2, the sulfur's valence electron = 6 And the valence electrons of oxygen = 6 There are 2 oxygen atoms in the compound, thus = 6*2 = 12 So, total valence electrons = 18 After drawing the skeletal structure, we can see that none of the atoms can fulfill their octet with single bonds. So there is a need for a double bond.
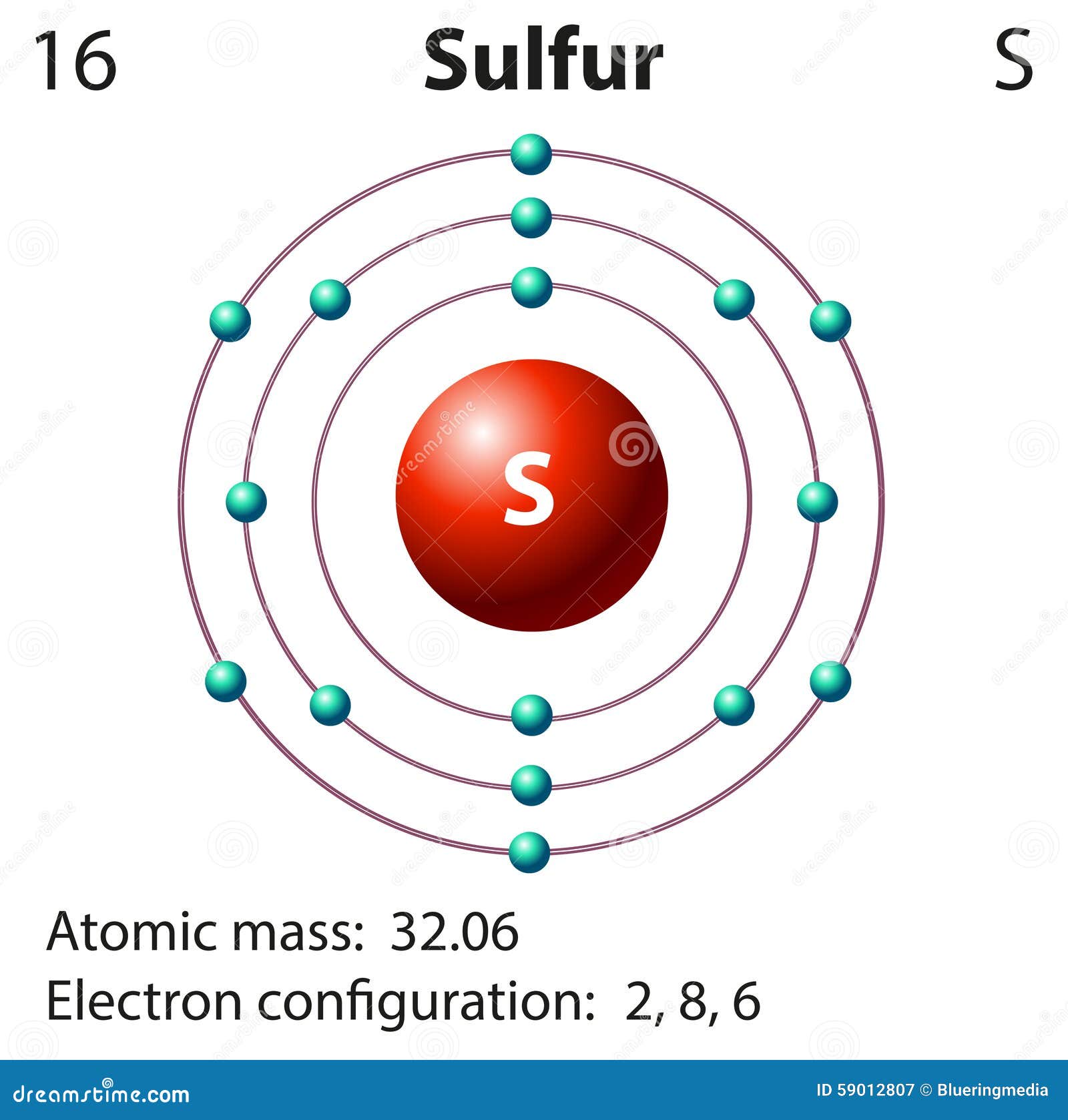
On the other hand, the atomic number of sulfur is 16 and the electronic configuration is 1s2 2s2 2p6 3s2 3p4. As p shell can accommodate 6 electrons, there is a dearth of two electrons, the ones in the 3s and 3p shells together forms the total number of valence electrons which is 6.
The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur S. It has bent or v shape. To draw the SO2 Lewis structure we have to find out the SO2 valence electrons firstWe express valence electrons as dots in lewis dot structure.
Sulfur has six valence electrons. Fluorine has seven valence electrons, but as there are six Fluorine atoms in this molecule, we will multiply this number by 6. = 6 + 7*6 = 6 + 42 = 48 valence electrons Thus SF6 has 48 valence electrons that will help us draw the Lewis Dot Structure of SF6. SF6 Lewis Structure
12+ Sulfite Lewis Structure. What is the lewis structure of sulfite? Can someone explain to me how dr. 6 Sulfur Trioxide - YouTube from i.ytimg.com. Lewis, who introduced it in his sulfites or sulphites are compounds that contain the sulfite ion so32−. Of course this is a resonance structure, in that all the sulfur oxygen bonds are equivalent.
I am in a normal Chemistry class in high school. I love science, and I really love biology, but I just can't grasp some of these concepts in chemistry. I've skimmed by every quarter, a C- every time. Ive tried so hard to understand it but it seems pointless. The biggest parts I don't understand is (in order from; no clue - kind of get it) A. Stoichiometry B. Finding the molar mass, and concentration. An example being - if it takes 206 mL of 0.2 M NaOH to neutralize 250 mL of an HCl solution, ...
Here are a number of highest rated Pf3 Lewis Dot Structure pictures on internet. We identified it from well-behaved source. Its submitted by running in the best field. We receive this kind of Pf3 Lewis Dot Structure graphic could possibly be the most trending subject taking into account we allocation it in google improvement or facebook.
Mar 23, 2020 — Note: Sulfur is in Group 16 (sometimes called Group VI or 6A). Since it is in Group 6 it will have 6 valence electrons.
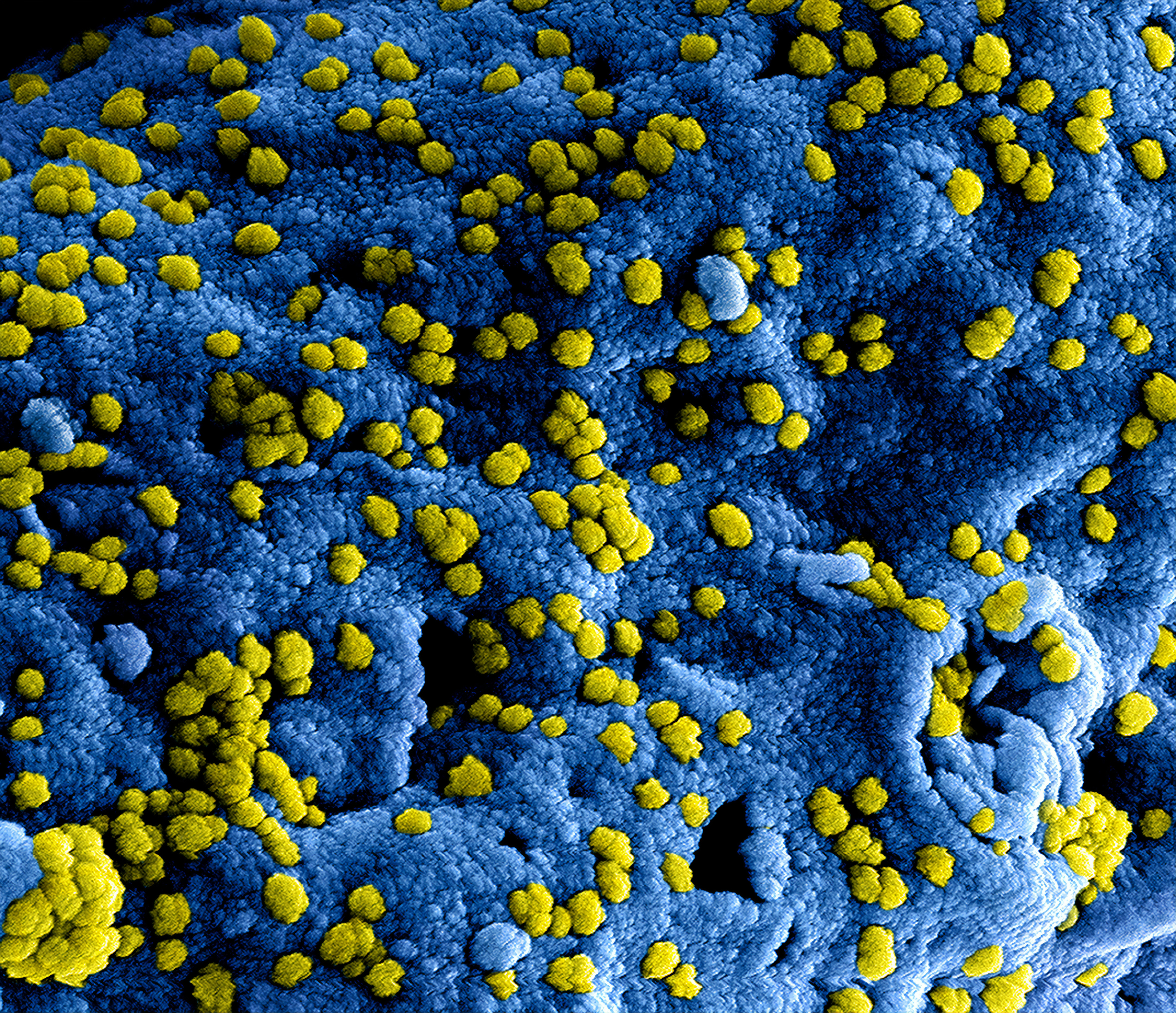
Produced by the National Institute of Allergy and Infectious Diseases (NIAID), this highly magnified, digitally colorized scanning electron microscopic (SEM) image, revealed ultrastructural details at the site of interaction of numerous yellow colored, Middle East respiratory syndrome coronavirus (MERS-CoV) viral particles, located on the surface of a Vero E6 cell, which had been colorized blue.
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
Again, it does not matter on which sides of the symbol the electron dots are positioned. For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. As usual, we will draw two dots together on one side, to represent the 2s electrons. However, conventionally, we draw the dots for the two p electrons on different sides. . As such, the …
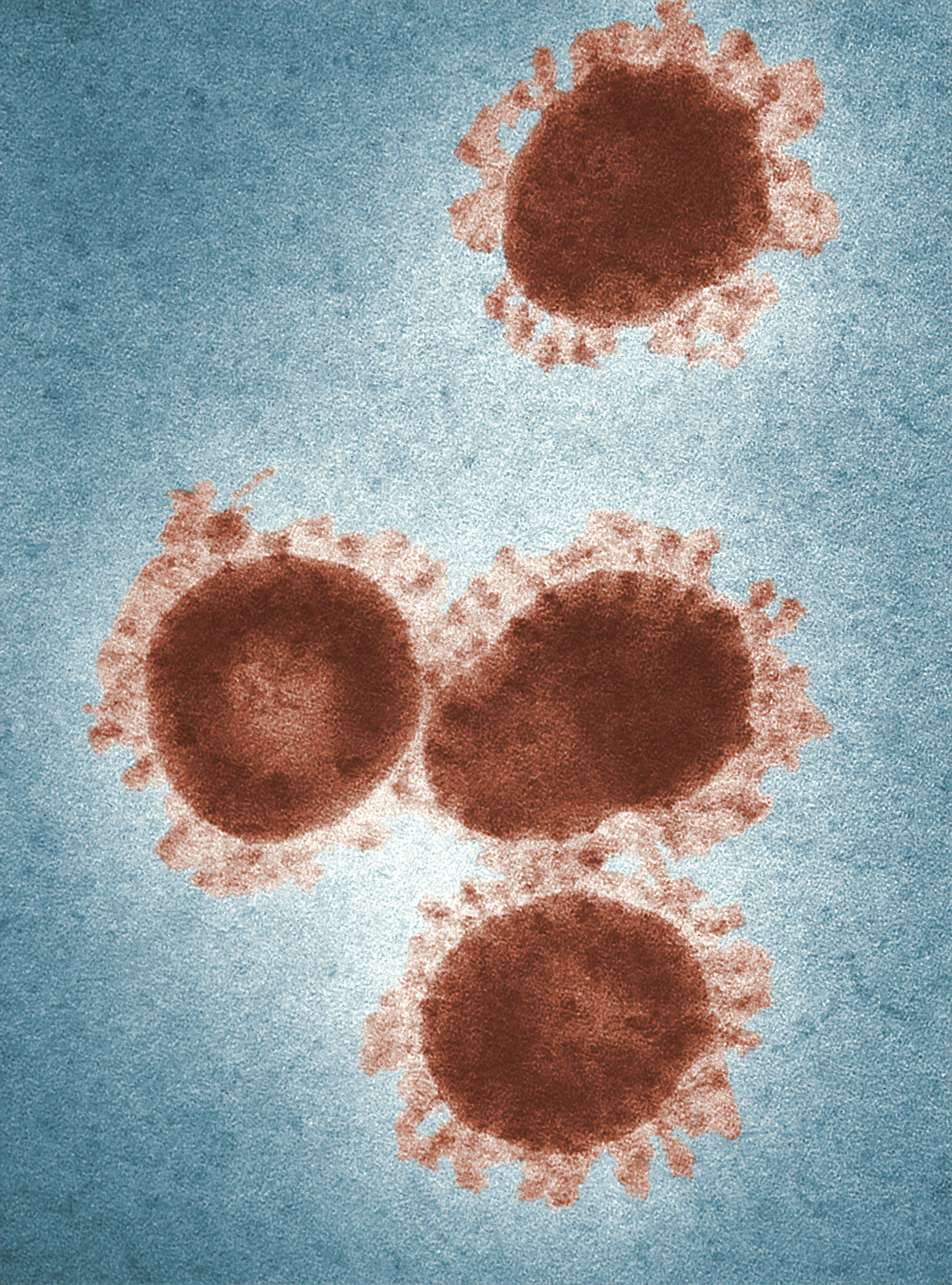
This 1975, digitally colorized transmission electron microscopic (TEM) image, depicted four avian infectious bronchitis virus (IBV) virions, which are Coronaviridae family members. IBV is a highly contagious pathogen, which infects poultry of all ages, affecting a number of organ systems, including the respiratory and urogenital organs. IBV possesses a helical genome, composed of non-segmented, positive-sense single-stranded RNA ((+) ssRNA). This is an enveloped virus, which means that its outermost covering is derived from the host cell membrane. The coronavirus derives its name from the fact
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron …
Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. There are three lone pairs on each fluorine atom. It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry.
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below.
SCl2 is used to synthesize other sulfur compounds like SOCl2, S4N4, S3H2, etc. In this article, we will understand the concepts of Lewis dot structure, hybridization, and polarity. We will also learn to determine the geometry and shape of a covalent compound using VSEPR theory. SCl2 Lewis Structure



















0 Response to "40 electron dot diagram for sulfur"
Post a Comment