37 tie line phase diagram
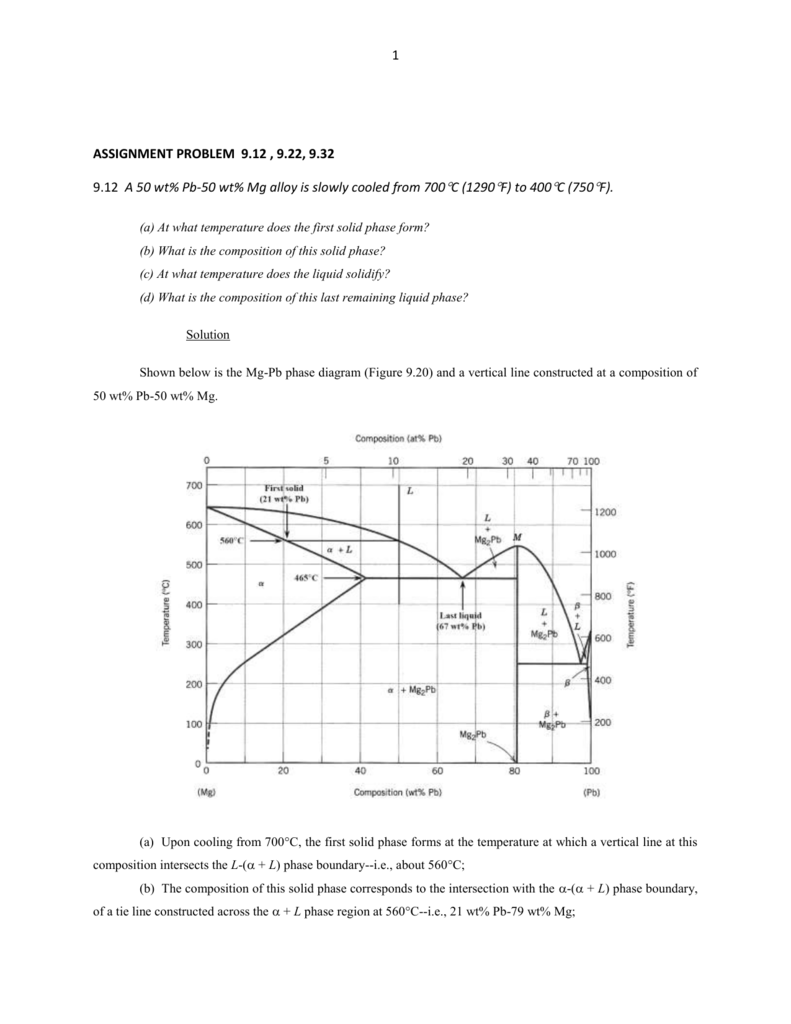
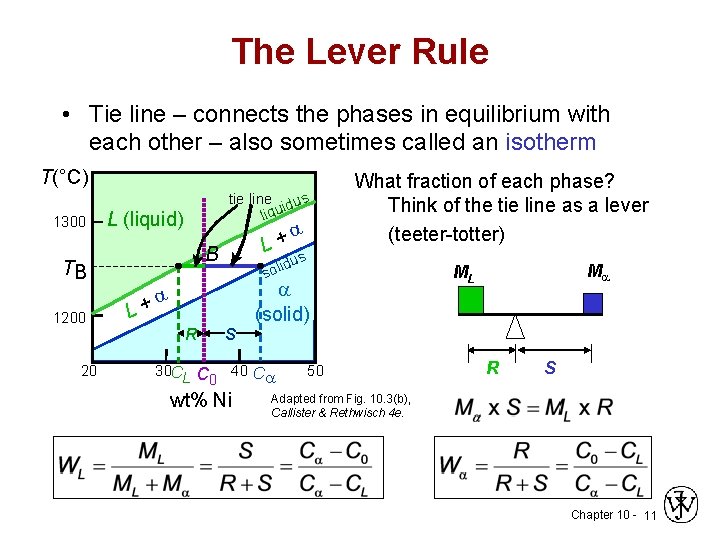
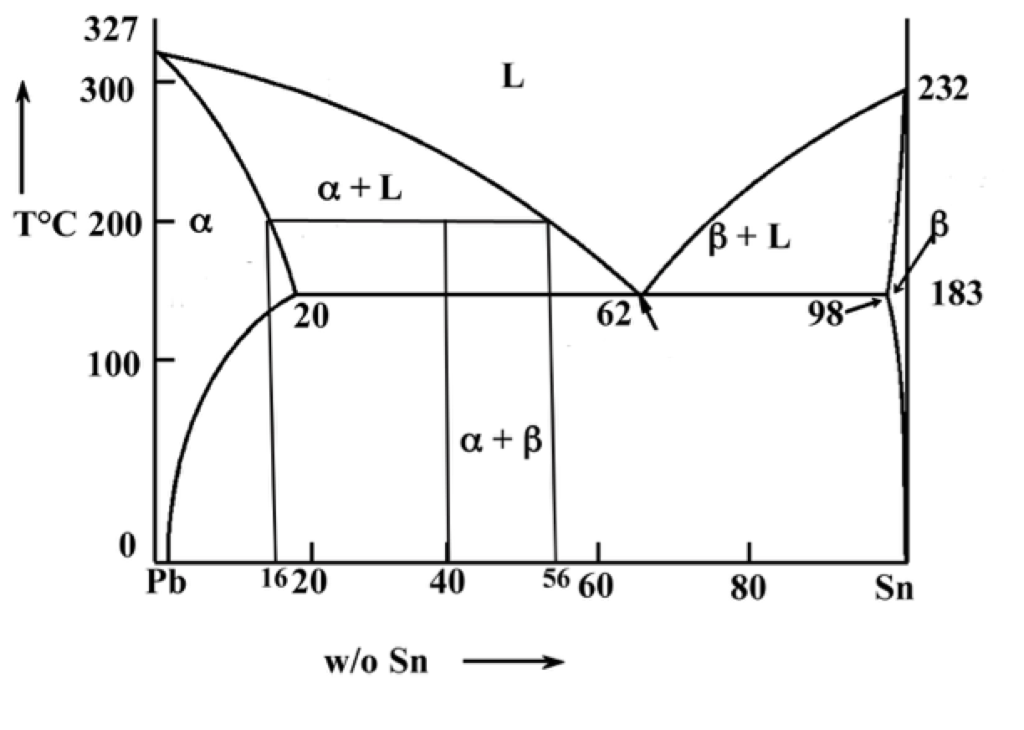
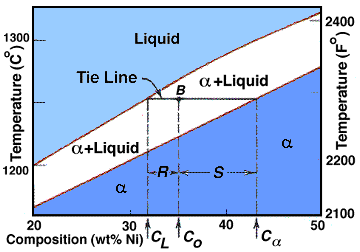
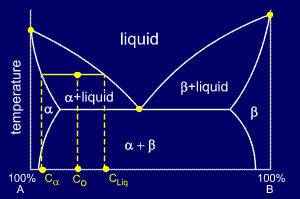
The tie line on a two-component solid- liquid phase diagram is an isothermal line that connects two equilibrium phases and passes through the alloy's position on the phase diagram when it is in a two-phase field, which intersects the two adjacent solubility curves. In a solid-liquid phase diagram, the solid-liquid phase boundary which is also ... in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
Phase Diagrams (Tie line, Lever Rule, Indentifcation of compositions and weight fractions in two-phase regions)Prof. Ratna Kumar Annabattula Department of Me...

Tie line phase diagram
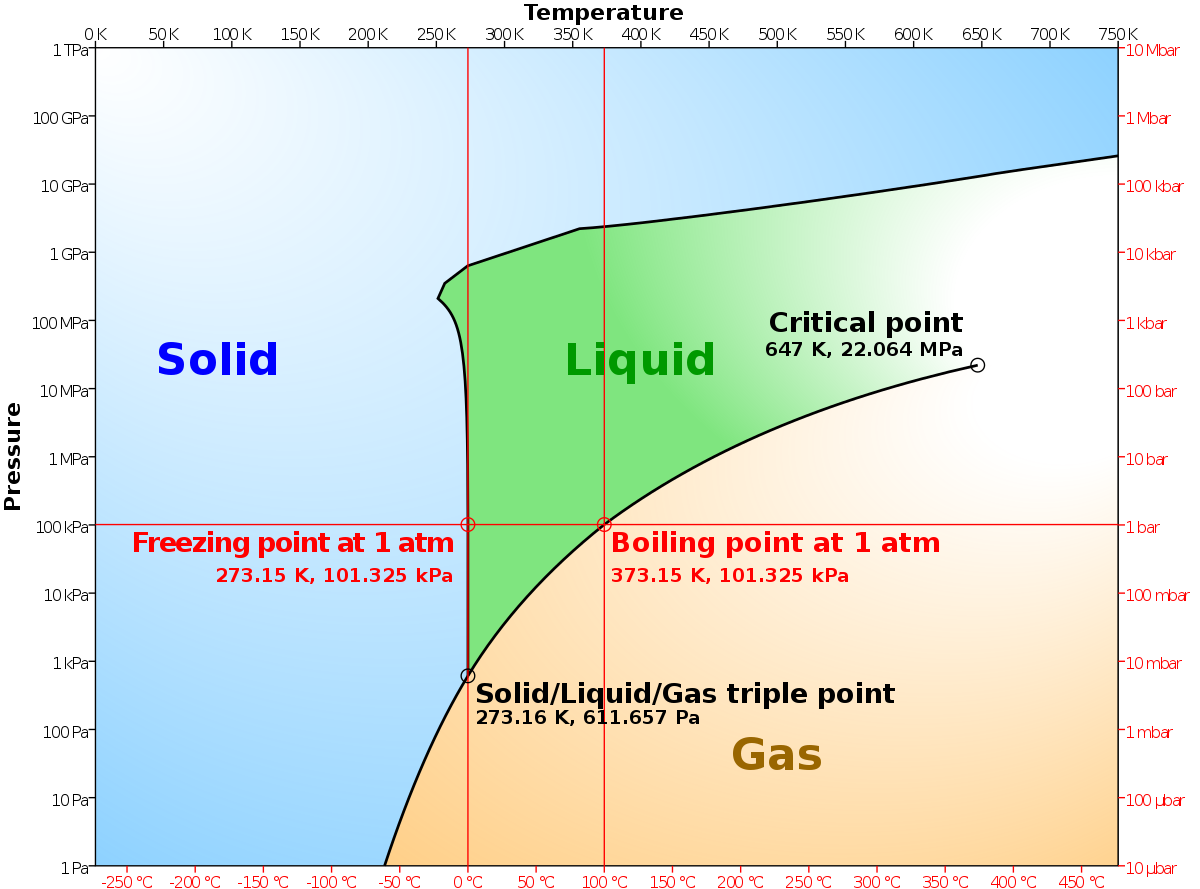
If you follow the line for say for 12 $^\circ$ C, from the degree notation there is at first one phases, a gas phase. Now when you reach the dotted curve there are two phases a gas phase and a liquid phase which are joined by a horizontal tie line. The whole thing is a lot easier to figure out with a more traditional phase diagram. represented in the portion of the Cu-Zn phase diagram shown below (at point A). Furthermore, the compositions of the phases, as determined from the tie line are Cε = 87 wt% Zn-13 wt% Cu Cη = 97 wt% Zn-3 wt% Cu Inasmuch as the composition of the alloy C0 = 90 wt% Zn, application of the appropriate lever rule expressions (for The tie lines determined in the two-liquid-phase coexistence region are found to be not parallel, suggesting that the dominant mechanism of lipid phase separation in this region changes with the membrane composition. We provide a phase diagram of the DOPC/eSM/Chol mixture and predict the location of the critical point.
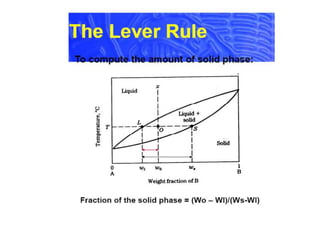
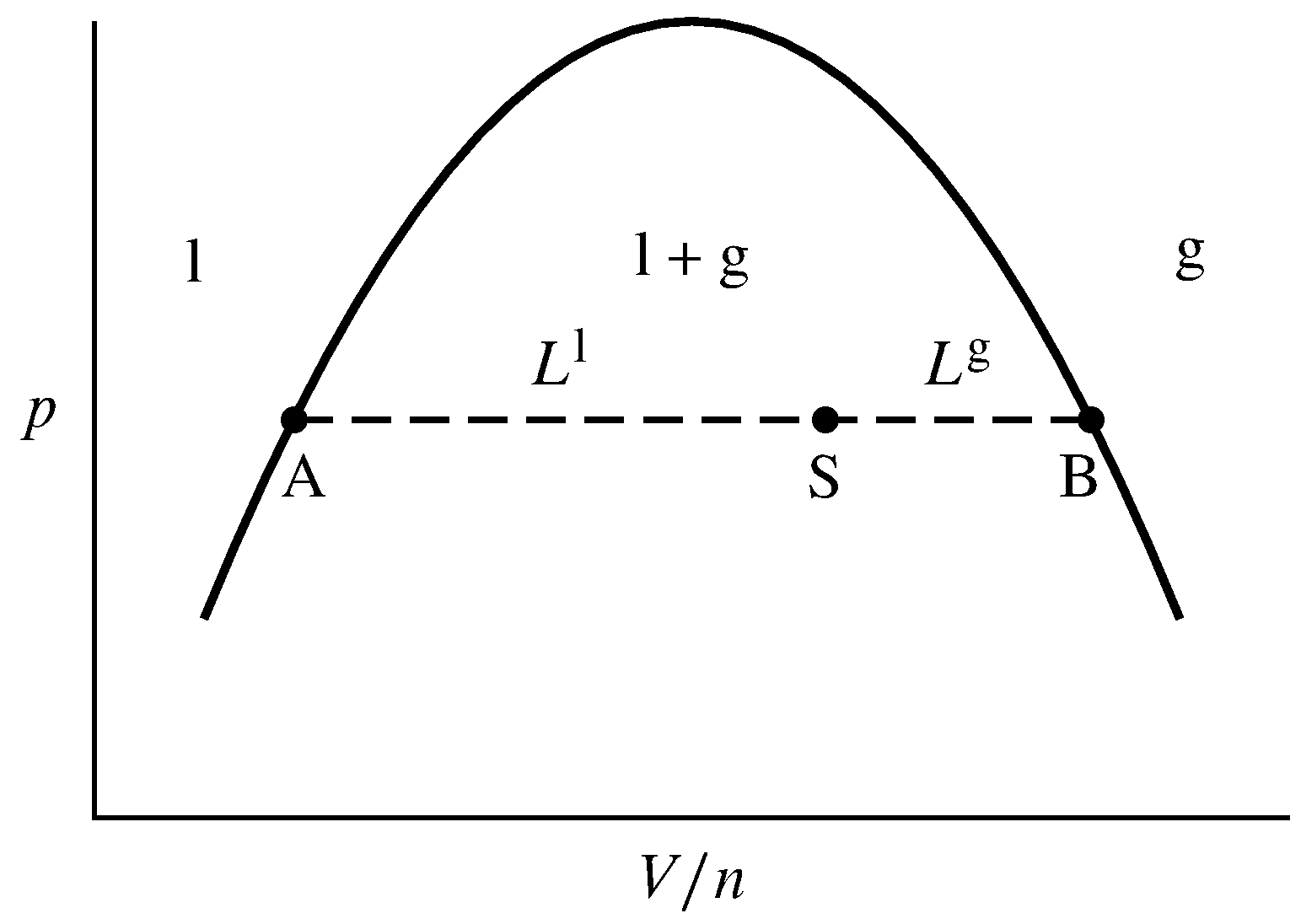
Tie line phase diagram. 6. A tie line is an isothermal (constant temperature) line drawn through the alloy's position on the phase diagram when it is in a two phase field. 6. Lever Rule 7. The points where the ends of the tie line intersect the two adjacent solubility curves indicate the compositions of the two phases that exist in equilibrium at this temperature. 8. A phase diagram with tie line (LS) for a binary isomorphous system. The x dimension defines the mass fraction of elements A and B. Binary phase diagrams Before any calculations can be made, a tie line is drawn on the phase diagram to determine the mass fraction of each element; on the phase diagram to the right it is line segment LS. Fig. 4 - Pressure-composition phase diagram for methane/butane and methane/decane binary systems at 160°F. As Fig. 3 shows, at 1,000 psia the two-phase region is a band that stretches from the C 1 -C 10 side of the diagram to the tie line on the C 1 -C 4 side. 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line The lever rule is a mechanical analogy to the mass balance calculation.
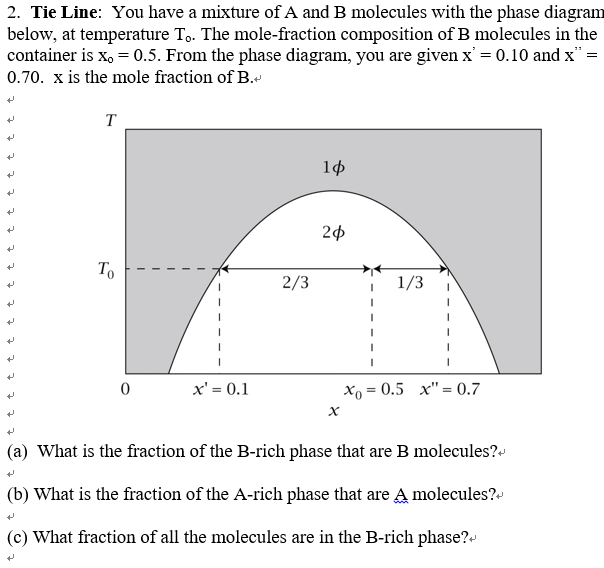
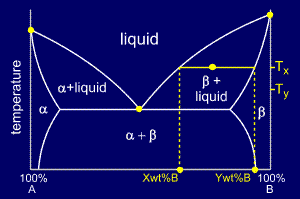
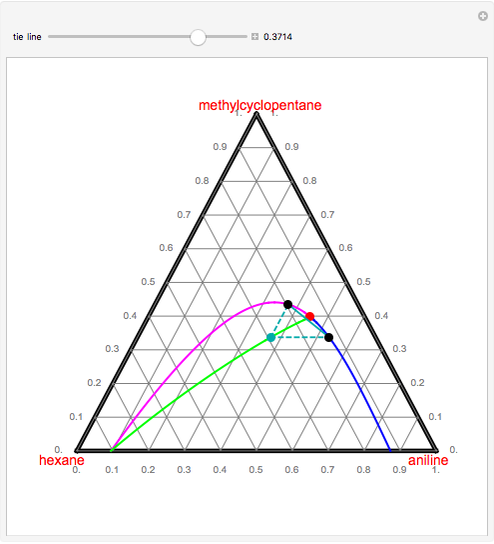
The point xN = 0.41, T = 290, occurs in the two-phase region of the diagram. The tie line indicates the phase boundaries at xN = 0.35 and xN = 0.83 (the compositions of the two liquid phases). The ratio of the amounts of each phase is equal to the ratio of the distances la and lb. Figure 2.1 (top) shows the methanol-chloroform-water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are shown in the diagram: Table 2.1 lists their respective compositions, and they all belong on the same tie-line. So, by definition, they separate into two liquid phases that have exactly the ... An isothermal(constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line(yes, that's the horizontal yellow line on the diagram). The ends of the tie lines show the compositions of the two As Fig. 8.12 shows, at 1,000 psia the two-phase region is a band that stretches from the C 1 -C 10 side of the diagram to the tie line on the C 1 -C 4 side. If the pressure is increased above 1,000 psia, the liquid composition line shifts to higher methane concentrations; methane is more soluble in both C 4 and C 10 at the higher pressure ...
The tie lines determined in the two-liquid-phase coexistence region are found to be not parallel, suggesting that the dominant mechanism of lipid phase separation in this region changes with the membrane composition. We provide a phase diagram of the DOPC/eSM/Chol mixture and predict the location of the critical point. phase diagrams,3-5,7,8,14 andthatisanother reasonwhyaccurate equilibrium phase diagrams could be valuable even if biological rafts consist of smaller, more transient entities that may not be in thermodynamic equilibrium. However, even when the regions ... If two samples are on the same tie-line, then the intrinsic Describes how to interpolate equilibrium tie lines on a ternary diagram. Made by faculty at the University of Colorado Boulder Department of Chemical and Bio... Tie line -Lever rule At a point in a phase diagram, phases present and their composition (tie-line method) along with relative fraction of phases (lever rule) can be computed. Procedure to find equilibrium concentrations of phases (refer to the figure in previous slide): - A tie-line or isotherm (UV) is drawn across two-phase
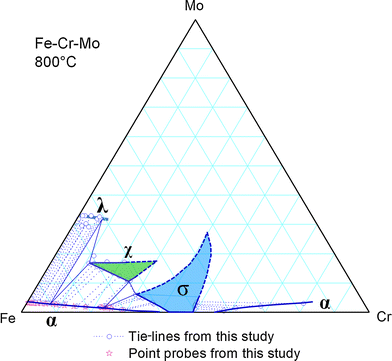
A tie line is specifically for a two-phase region, regardless of the dimension of the phase diagram (binary/ternary/quaternary). So in a 2-phase region, simply proceed as in the binary case. Coming...
Interpretation of Binary Phase Diagram - Using Phase Rule, Tie-Line and Lever Rule For a binary system of known composition and temperature that is at equilibrium, at least three kinds information are available: (1) the phases that are present, (2) the compositions of these phases, and (3) the percentages or fractions of the phases. For ...
An isothermal (constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line (yes, that's the horizontal yellow line on the diagram). Click to see full answer
People also ask, what is a tie line in phase diagram? An isothermal (constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line (yes, that's the horizontal yellow line on the diagram). Similarly, what is a ternary system?
An isothermal (constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line (yes, that's the horizontal yellow line on the diagram).
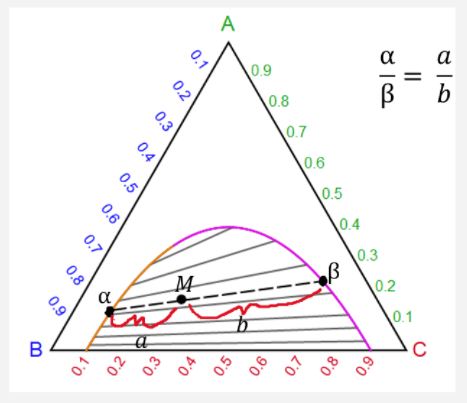
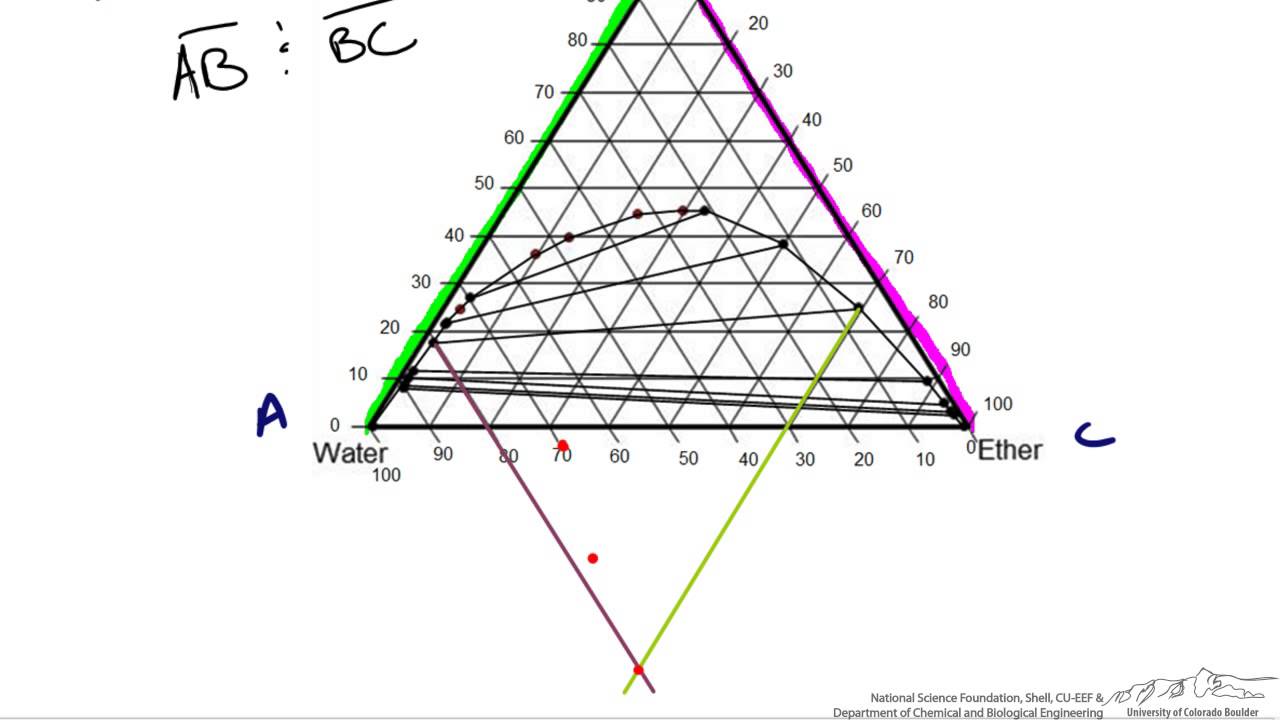
Tie Lines in Phase Diagrams for Ternary Liquid Systems Most laboratory programs in physical chemistry at the undergraduate level include an exercise in the construc- tion of a phase diagram, on triangular coordinate paper, for a ternary liquid system.
What is tie line in chemistry? (physical chemistry) A line on a phase diagram joining the two points which represent the composition of systems in equilibrium. Also known as conode. Click to see full answer. Thereof, what do tie lines represent?
Understanding Phase Diagrams - V.B. John . Ternary phase diagram books by D.R.F. West - there are several . Ternary grid . ... • Overall should lie on tie line of 2 phases, else - Phase missing - At least one inaccurate result - suspect smallest! • Overall should lie in tie triangle of 3 phases
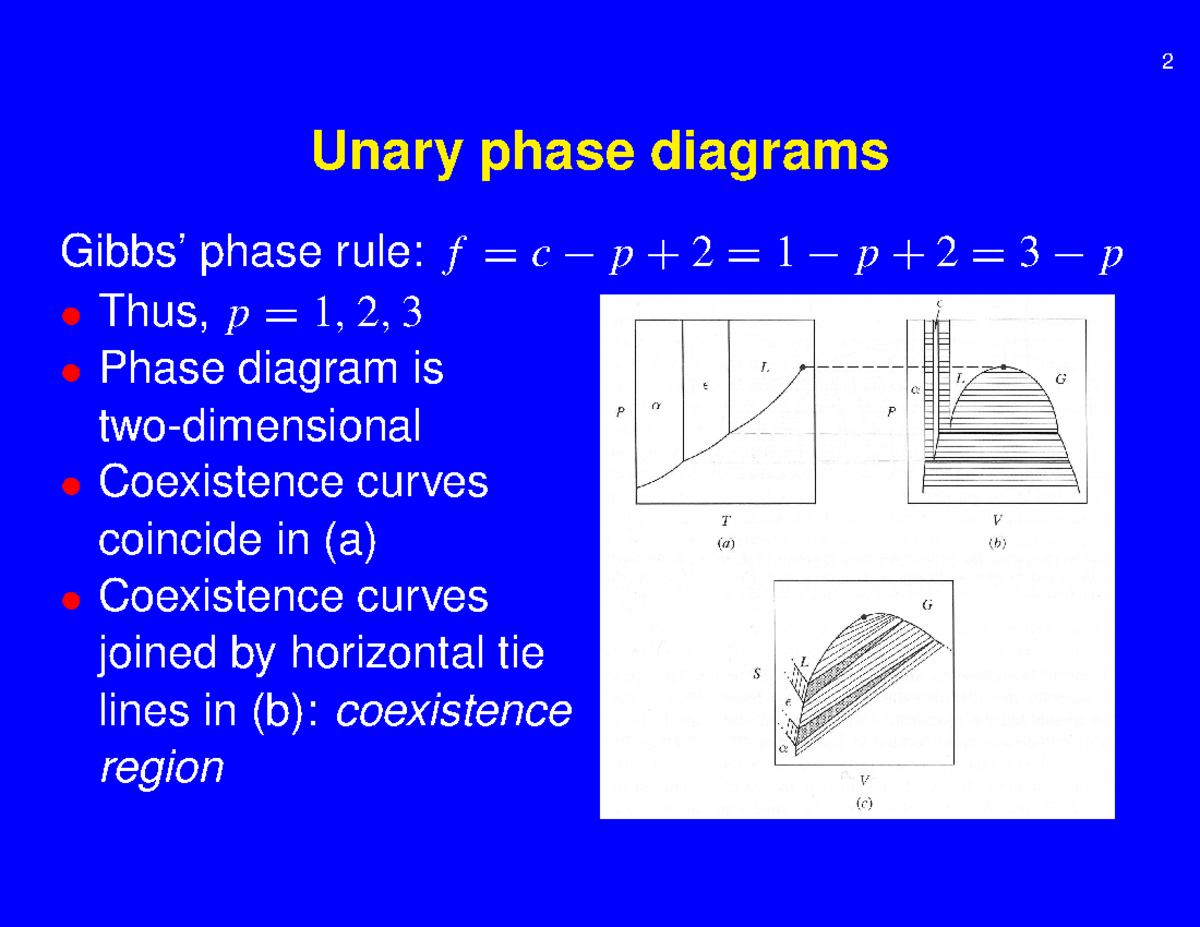
If the entire length of the tie line mo is taken to represent 100% or the total weight of the two phases present at temperature T, the lever rule may be expressed mathematically as: For such phase diagram, there is one degree of freedom. So, in this case Gibb's rule converts into
1. Locate composition and temperature in diagram . 2. In two phase region draw the tie line or isotherm . 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line . The lever rule is a mechanical analogy to the mass balance calculation. The ...
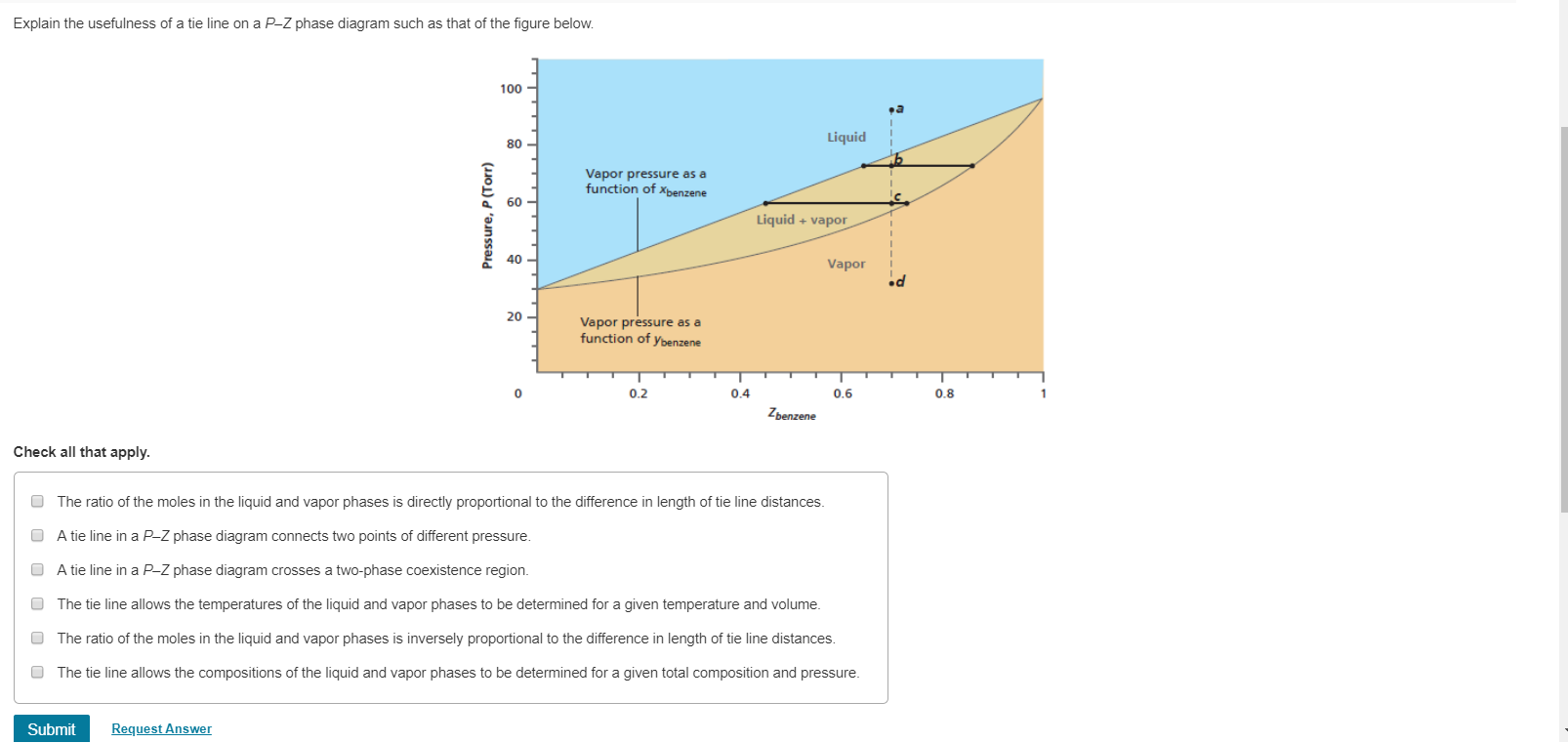
Click to see full answer Thereof, what is a tie line in ternary phase diagram? Tie lines connect compositions of liquid and vapor phases in equilibrium.Any mixture with an overall composition along a tie line gives the same liquid and vapor compositions. Only the amounts of liquid and vapor change as the overall composition changes from the liquid side of the binodal curve to the vapor side.
The tie lines determined in the two-liquid-phase coexistence region are found to be not parallel, suggesting that the dominant mechanism of lipid phase separation in this region changes with the membrane composition. We provide a phase diagram of the DOPC/eSM/Chol mixture and predict the location of the critical point.
represented in the portion of the Cu-Zn phase diagram shown below (at point A). Furthermore, the compositions of the phases, as determined from the tie line are Cε = 87 wt% Zn-13 wt% Cu Cη = 97 wt% Zn-3 wt% Cu Inasmuch as the composition of the alloy C0 = 90 wt% Zn, application of the appropriate lever rule expressions (for
If you follow the line for say for 12 $^\circ$ C, from the degree notation there is at first one phases, a gas phase. Now when you reach the dotted curve there are two phases a gas phase and a liquid phase which are joined by a horizontal tie line. The whole thing is a lot easier to figure out with a more traditional phase diagram.











0 Response to "37 tie line phase diagram"
Post a Comment