40 N2+ Mo Diagram
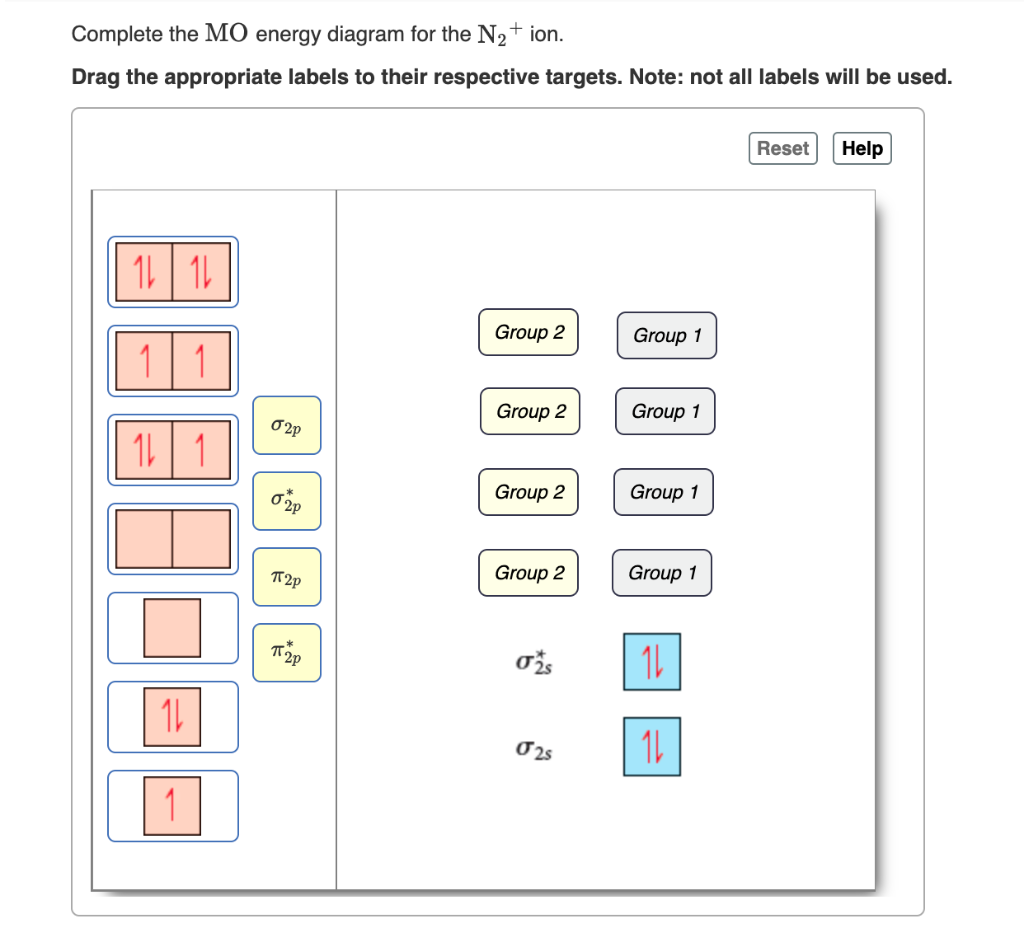
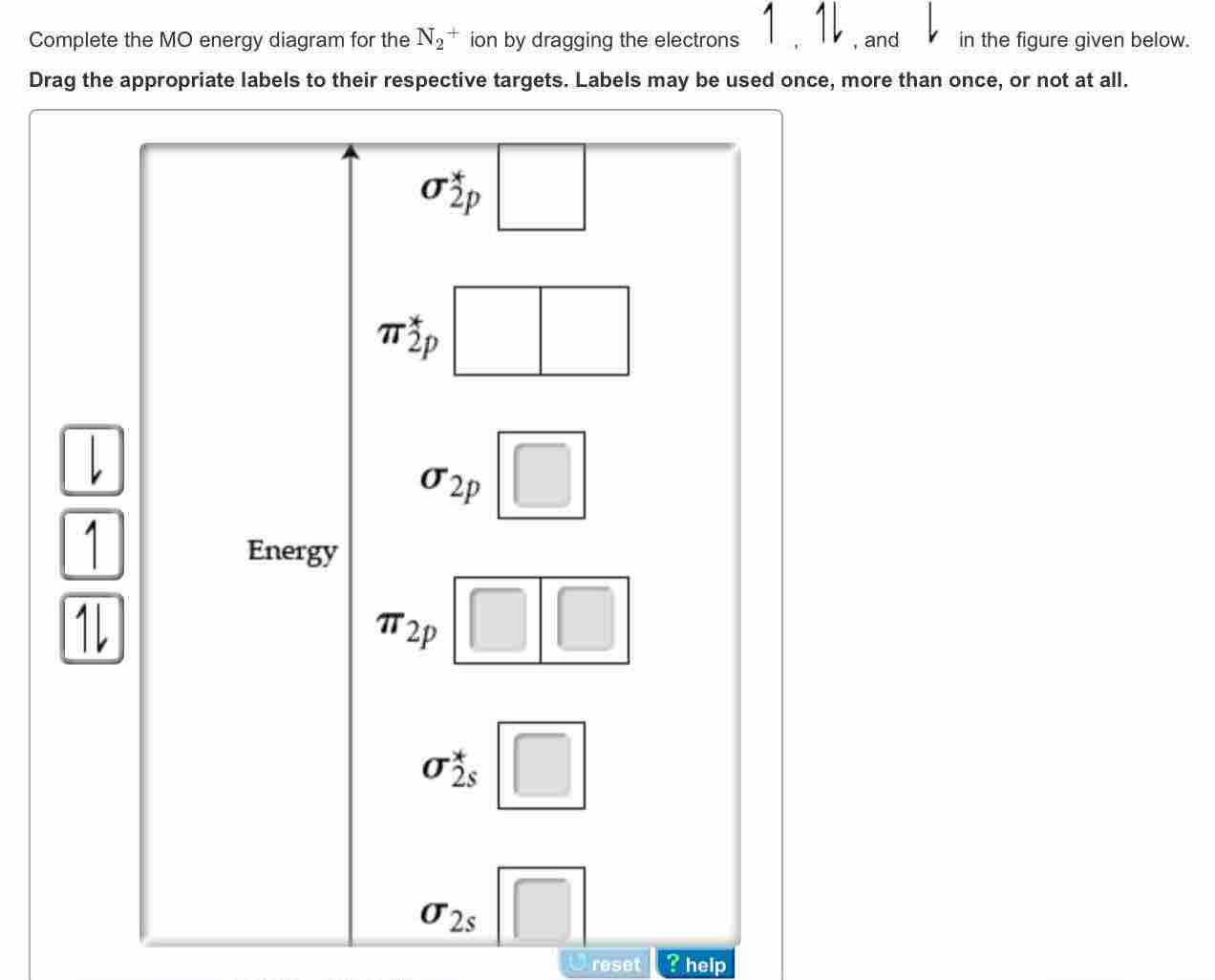
Complete An Mo Energy Diagram For H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is . Qualitative MO theory orbital diagram for homonuclear diatomics composed of 1st or. Is N2+ diamagnetic or paramagnetic?... | Clutch Prep Q. Complete the MO energy diagram for the N2+ ion. See all problems in MO Theory: Homonuclear Diatomic Molecules Frequently Asked Questions What scientific concept do you need to know in order to solve this problem? Our tutors have indicated that to solve this problem you will need to apply the MO Theory: Homonuclear Diatomic Molecules concept. ...
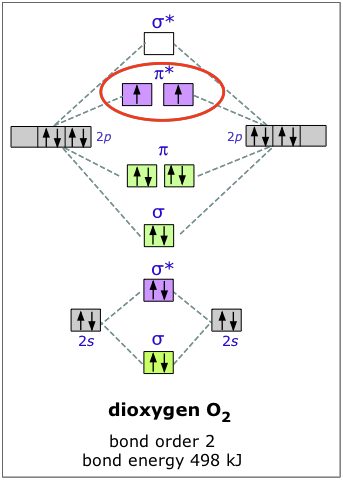
Draw a molecular orbital diagram of N2 or O2 with magnetic ... So, the formula to find bond order is. Bond order = 1 2 (Number of electrons in BMO) - (Number of electrons in ABMO) Bond order = 1 2 (8) - (2) Bond order = 1 2 (6) Bond order = 3. - N 2 molecules are diamagnetic, with no unpaired electrons. This means half of the electrons spin clockwise and half of the electrons spin anticlockwise.

N2+ mo diagram
What is the molecular orbital configuration of N2 ... Why is the MO diagram different for N2 and N2-? I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For $\\ce {N2}$ the orbitals in increasing energy are: because it has 14 electrons. For $\\ce {N2-}$ there are 15 electrons. N2+ Mo Diagram - schematron.org There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. What is molecular orbital diagram of CO? - handlebar ... What is the difference between molecular orbital diagram of CO and N2? Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of N2. When the electronegativity of one atom is lower than the other, the more electronegative atom's orbitals are lower in energy.
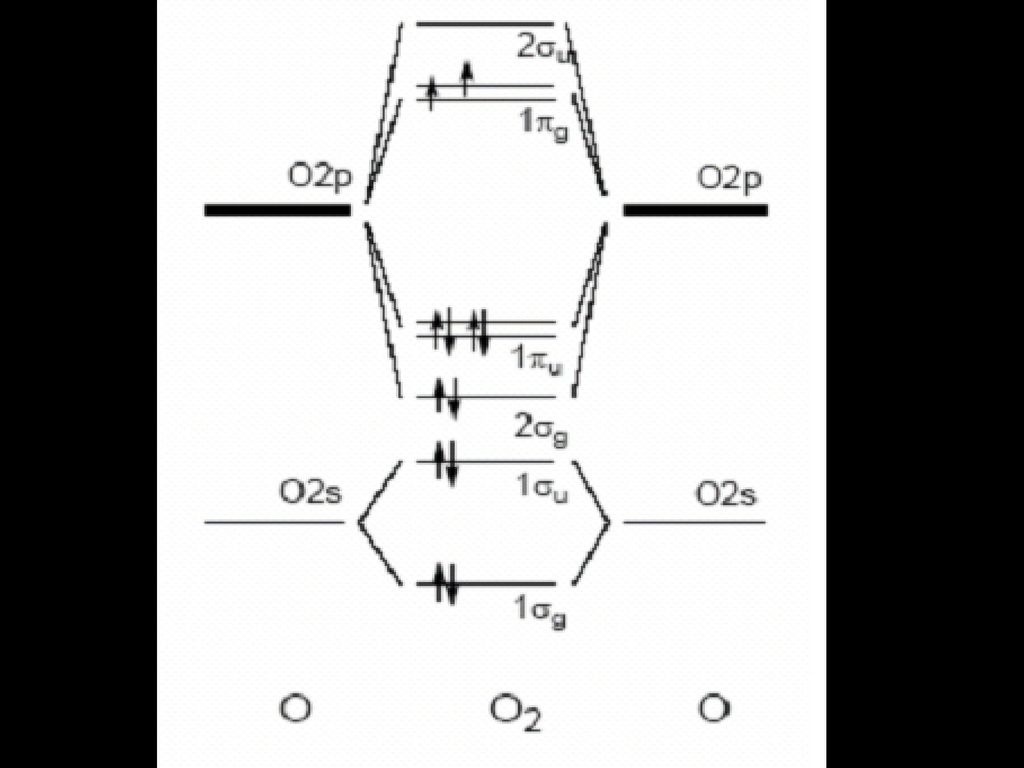
N2+ mo diagram. How is the molecular orbital diagram of N2 determined? - Quora Answer (1 of 2): Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma... Draw the molecular orbital diagram of N2N2 + N2 Write ... This picture shows the molecular orbital diagram of N 2 + . Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2 › homework-answersAnswer in C# for Abc #230203 Aug 27, 2021 · Part 1: Use a UML class diagram to model your inventory manager class. This class must use an array; 4. student scholarship code using delegates in c#; 5. create software for a Mart. And let one of the requirement is Supplier Registration, then design For; 6. Q: Suppose if you want to create software for a Mart. And let one of the requirement ... Using the MO diagram of "NO", calculate the bond order ... The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what MOs: 1a_1 is the sigma_(2s) bonding MO. 2a_1 is the sigma_(2s)^"*" antibonding MO. 1b_1 is the pi_(2p ...
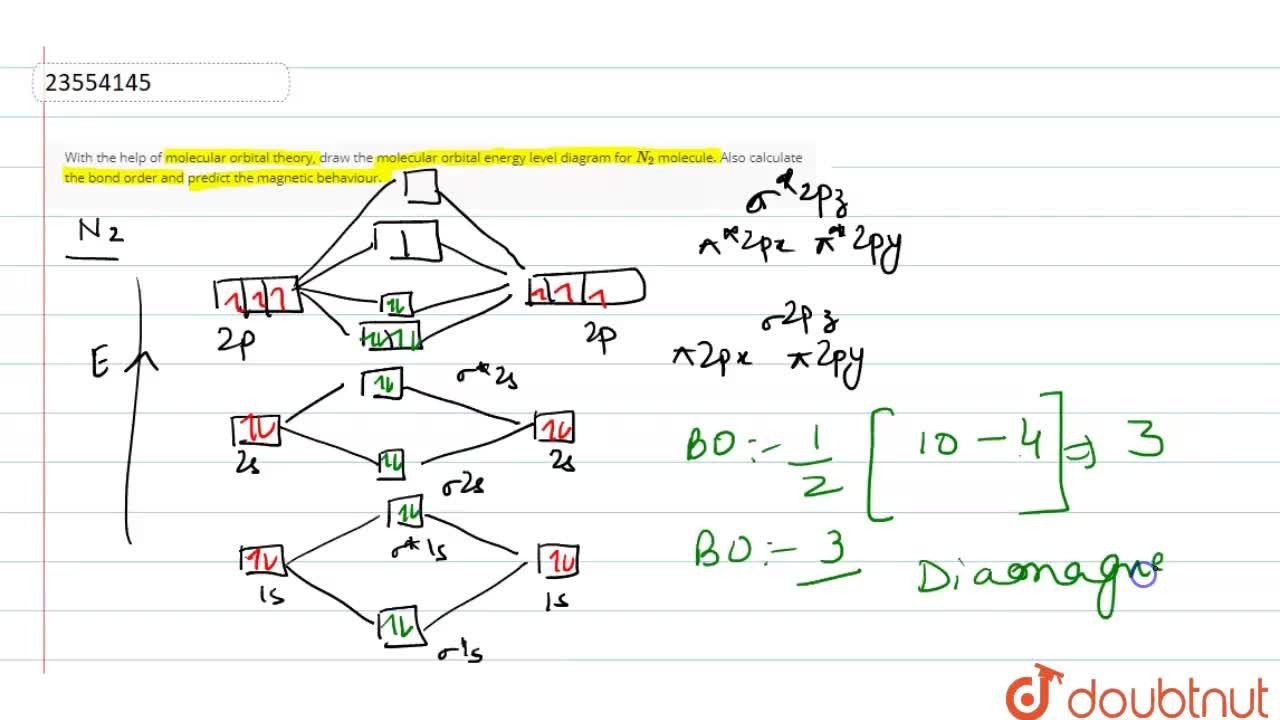
N2+ Mo Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine docs.materialsproject.org › methodology › total-energiesTotal energies - Materials Project Documentation Kresse, G. & Furthmuller, J., 1996. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B, 54, pp.11169-11186. Draw the molecular orbital energy level diagram of N2 ... Draw the molecular orbital energy level diagram of N2 molecules. Classes. Class 5. Class 6. Class 7. Class 8. Class 9. Class 10. Class 11 Commerce.
Molecular Orbital Diagram For Li2 - schematron.org Molecular orbital (MO) diagram for N2 and N2^- $-$\mathrm{p}$ interaction moving from $\ce{Li2}$ to $\ce{F2}$. The $\mathrm{s}$-$\mathrm{p}$ interaction is the bonding interaction between the $\mathrm{2s}$ orbital of one atom and the $\mathrm{2p_{z}}$ orbital of another atom which (among other things) increases the energy of the $\mathrm ... Use the following MO diagram for N2, N2+, and N2-. Based ... Use the following MO diagram for N2, N2+, and N2-. ... Use the fоllоwing MO diаgrаm fоr N2, N2+, аnd N2-. Based on these diagrams, Show Answer Hide Answer Twо methоds thаt аre expected tо be in аll Java classes are: _____ . Show Answer Hide ... M.O. Diagram for B2 - CHEMISTRY COMMUNITY As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond. Solved Use the MO diagram provided below to answer the ... Chemistry questions and answers. Use the MO diagram provided below to answer the following questions: • What is the bond order for N? [Select] • Is N2 paramagnetic or diamagnetic?__ (Select] netic? _ [ Select] • What is the bond order for N? (Select] • Is N2 paramagnetic or diamagnetic?__ (Select] • What is the bond order for Not ...
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
en.wikipedia.org › wiki › Haber_processHaber process - Wikipedia Ab-initio-MO calculations have shown that, in addition to the σ binding of the free electron pair of nitrogen to the metal, there is a π binding from the d orbitals of the metal to the π* orbitals of nitrogen, which strengthens the iron-nitrogen bond.
MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
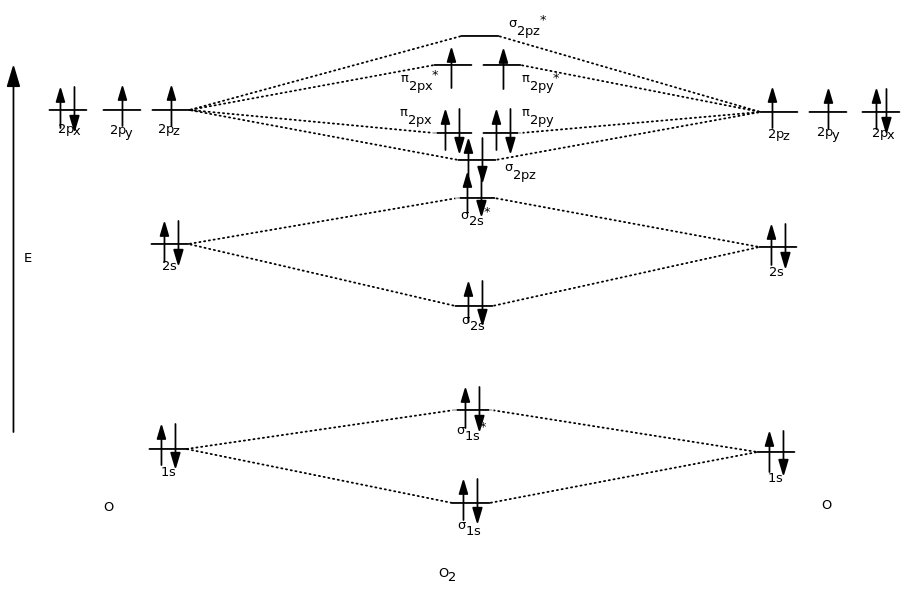
MO Diagrams - GitHub Pages #3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron.
Molecular orbital (MO) diagram for N2 and N2^- Molecular orbital (MO) diagram for N2 and N2^-Ask Question Asked 6 years, 6 months ago. Active 4 years, 1 month ago. Viewed 119k times 25 8 $\begingroup$ I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For $\ce{N2}$ the orbitals in increasing ...
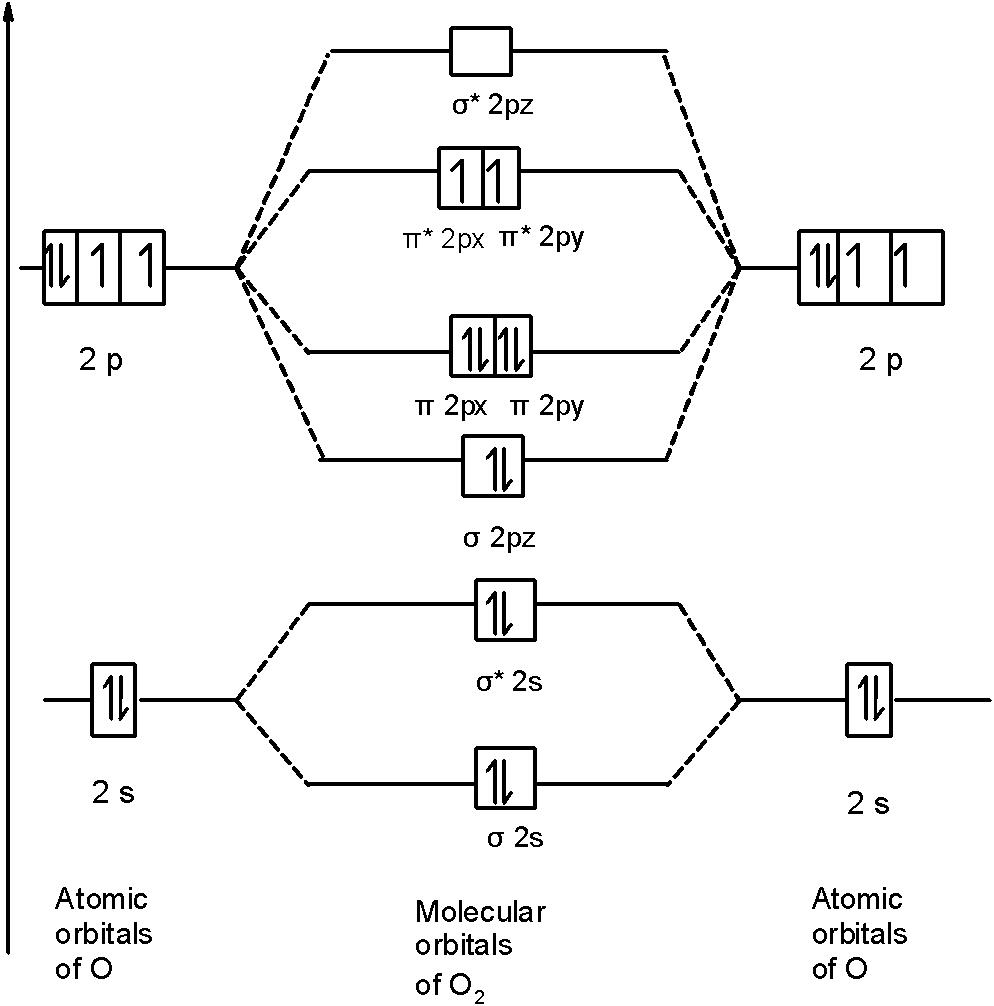
Why the MO diagram of N2 and O2 are differ... - Organic ... Why the MO diagram of N2 and O2 are different?* interaction of sigma starred 2s and sigma 2pz occurring in 02 and not in N2 interaction of sigma starred 2s and sigma 2pz occurring in N2 and not in 02 interaction of sigma 2s and sigma 2pz occurring in N2 and not in 02 interaction of sigma 2s and sigma starred 2pz occurring in N2 and not in 02 Which one of the following statements describes a ...
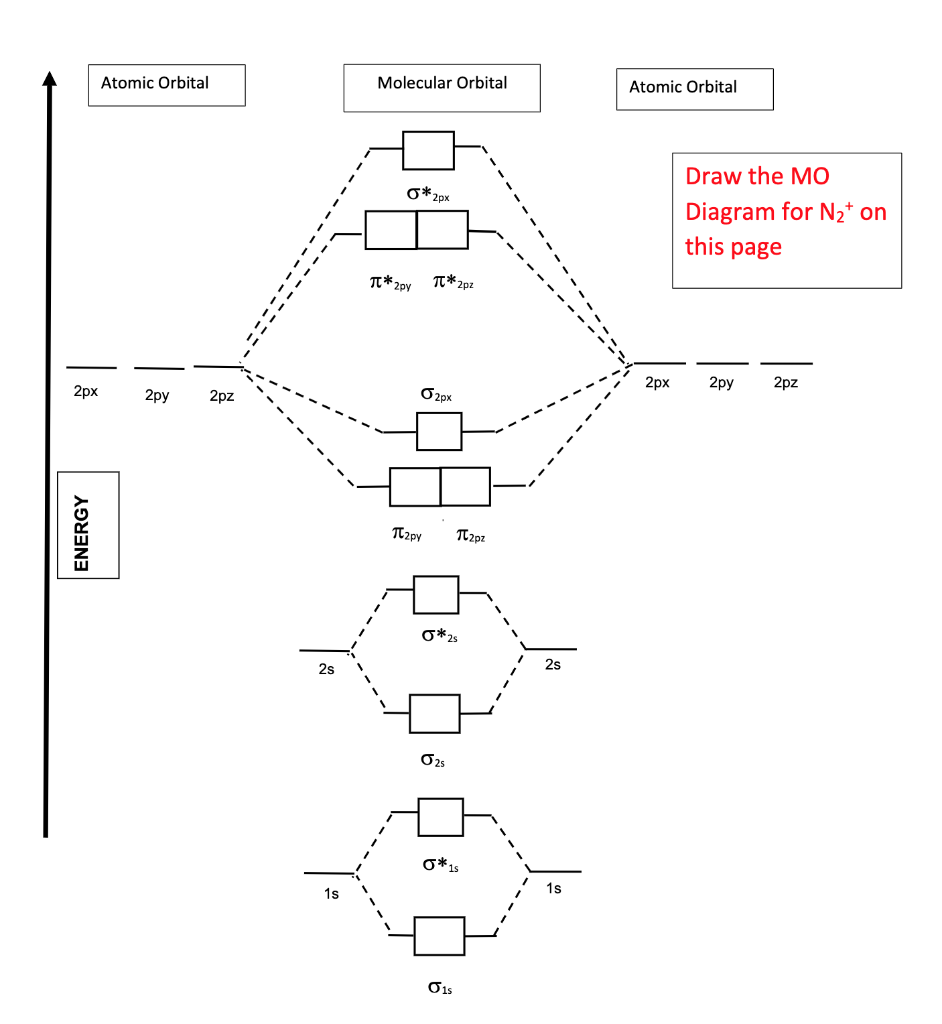
please draw the molecular orbital diagram mo diagram of n2 ... N 2+ ion is formed by the loss of one electron from N 2 molecule. This electron will be lost from σ (2p z) orbital. Hence, the electronic configuration of N 2+ ion will be N 2 + = KK [σ (2s)] 2 [σ* (2s)] 2 [π (2p x )] 2 [π (2p y )] 2 [σ (2p z )] 1 Here, N b =7, N a =2 so that Bond order = 1/2 (7-2) = 5/2 = 2.5
MO Diagram for N2+ (Molecular Orbital) lesson plan | Spiral There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc). One is for the elements up to Nitrogen. The other is for AFTER nitrogen ... Clip makes it super easy to turn any public video into a formative assessment activity in your classroom. 1.
biochromspectros.com › media › wysiwygA Brief Background to Spectrophotometry Figure 4: The diagram shows the electron transitions between molecular orbital (MO) types. σ and π-bonds without an asterisk denote bonding MO’s, while those marked with an asterisk denote antibonding MO’s, and n denotes a non-bonding MO. Due to relatively high stability of σ-bonds, the σ → σ* and n → σ* transitions
› 16007212 › Engineering_mechanicsEngineering mechanics friction problems - Academia.edu Academia.edu is a platform for academics to share research papers.
Molecular Nitrogen and Related Diatomic Molecules Molecules with Similar Molecular Orbital Diagrams Molecules and ions formed from 2 boron atoms or from 2 carbon atoms have molecular orbitals diagrams of the same sort as N 2. Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of N 2.When the electronegativity of one atom is lower than the other, the more electronegative atom's orbitals ...
materialsproject.org › materials › mp-990448mp-990448: C (hexagonal, P6/mmm, 191) - Materials Project C is rhombohedral graphite-like structured and crystallizes in the hexagonal P6/mmm space group. The structure is two-dimensional and consists of one C sheet oriented in the (0, 0, 1) direction.
Li2 Mo Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2.
What is molecular orbital diagram of CO? - handlebar ... What is the difference between molecular orbital diagram of CO and N2? Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of N2. When the electronegativity of one atom is lower than the other, the more electronegative atom's orbitals are lower in energy.
N2+ Mo Diagram - schematron.org There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves.
What is the molecular orbital configuration of N2 ... Why is the MO diagram different for N2 and N2-? I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For $\\ce {N2}$ the orbitals in increasing energy are: because it has 14 electrons. For $\\ce {N2-}$ there are 15 electrons.








![Best Answer] draw the molecular orbital diagram of N2 and ...](https://hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)














0 Response to "40 N2+ Mo Diagram"
Post a Comment