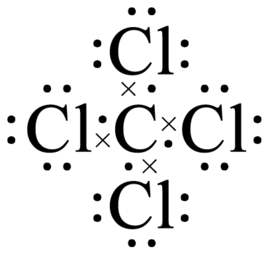
41 lewis diagram for ccl4
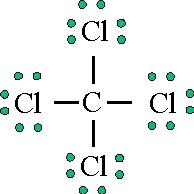
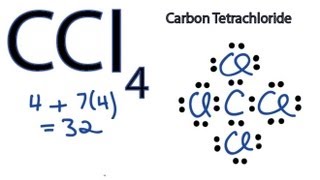
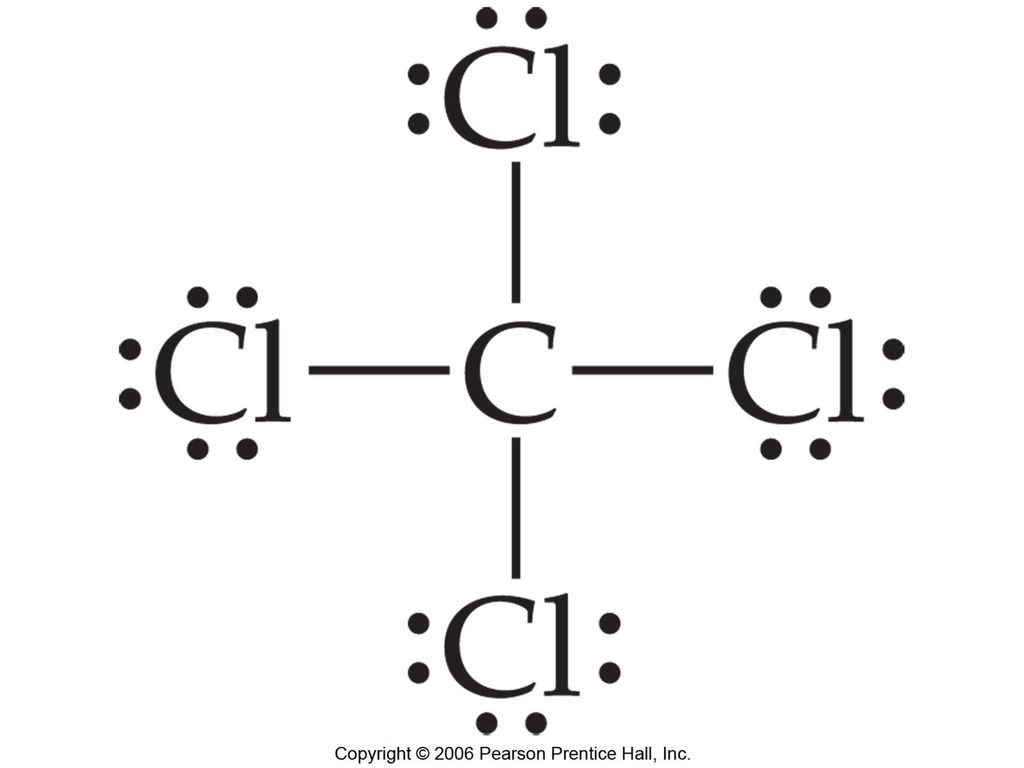
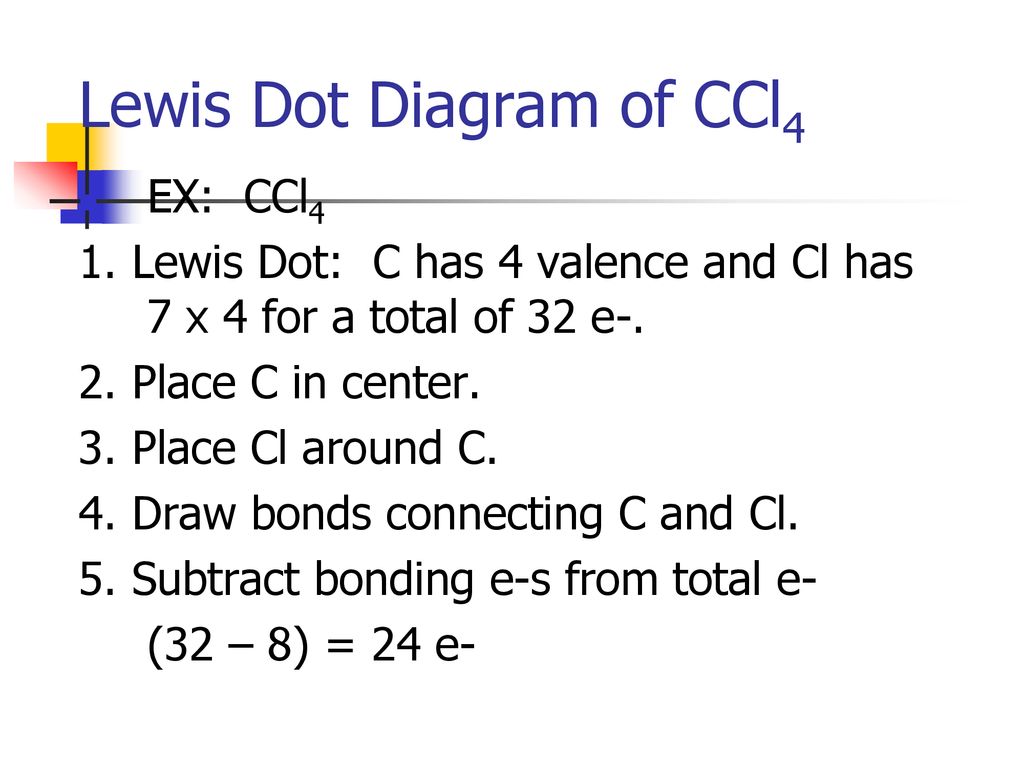
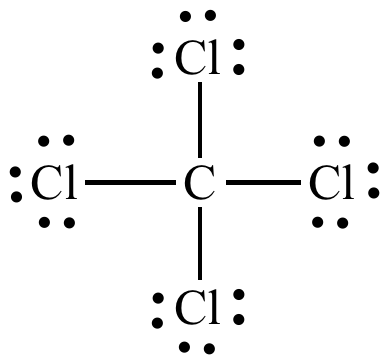
Lewis Dot Structure for CCl4 The Lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. The electrons are represented with the help of circular dots. This diagram displays the bonds formed as well as lone pairs of electrons. Consider the diagram given above. For the Lewis structure of CCl4 first, let’s calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly. = 4 + (4*7) = 4 + 28 = 32 valence electrons
The hybridization of CCl4 is sp3. This helps us to understand the geometry of CCl4 which is tetrahedral. The bond angle between the atoms is somewhere around 109 degrees. This is all about the compound CCl4, its Lewis structure, hybridization, molecular geometry, polarity, applications, and MO diagram.

Lewis diagram for ccl4
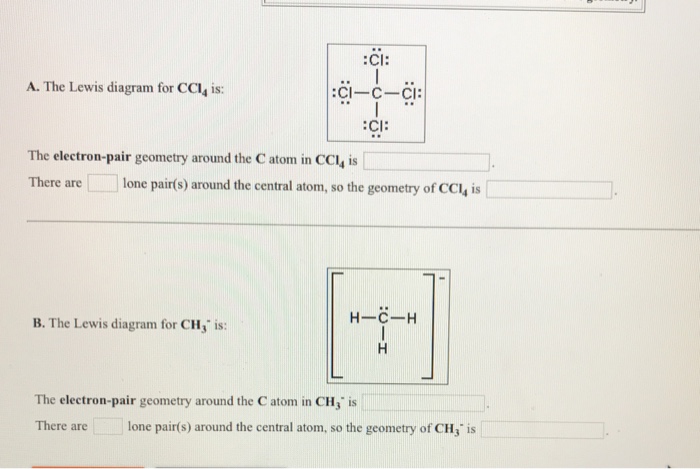
Nov 12, 2018 · A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over hybridization, shape and bond angle. The Lewis diagram for CC4 ::Cl-c-Ci: :CI: The electron-pair geometry around the C atom in CCl4 is There arelone pair(s) around the central atom, so the geometry of CCl4 is CI: B. The Lewis diagramh for AICI3 is: AI The electron-pair geometry around the Al atom in AICl, is There arelone pair(s) around the central atom, so the geometry of AICl, is
Lewis diagram for ccl4. Lewis diagram for ccl4 Lewis dot diagram for ccl4. Hi, today I'm going to design the CCl4 Lewis structure for CCl4 in just four steps. CCl4 Lewis Structure Setup Step-1: To design the CCl4 Lewis structure (carbon tetrachloride), we must first discover the electrons of CC4 valence. The Lewis dot structure for CCl4 starts with a C in the middle. Four dashes are drawn, one on each side, each connecting to a Cl atom. On the unconnected sides of the Cl atoms, there are two dots ... Problem: Draw the Lewis structure for CCl4. FREE Expert Solution We're being asked to draw a Lewis structure for CCl 4. To do so, we first need to do the following steps: Step 1: Determine the central atom in this molecule. Step 2: Calculate the total number of valence electrons present. Answer: Since carbon may have 4 bonds (it has 4 valence electrons), it will bond with the 4 Chloride ions. The dipoles at opposite sides will cancel out (top to bottom & left and right), thus the shape will have 4 equally angled bonds by VSEPR theory and have a tetrahedral structure.
For this problem, we need to do the following steps: Step 1: Determine the central atom in this molecule. Step 2: Calculate the total number of valence electrons present. Step 3: Draw the Lewis structure for the molecule. Step 4: Draw dipole arrows for each bond. Step 5: Determine the polarity of the molecule. Step 1: Carbon is less electronegative than chlorine so carbon is the central atom. Follow some steps for drawing the lewis dot structure of CBr4 1. Count total valence electron in CBr4 Finding the total number of valence electrons in the CBr4 molecule is the first step for drawing its lewis diagram. "A valence electron is the outermost shell electrons around an atom". Answer: Since carbon may have 4 bonds (it has 4 valence electrons), it will bond with the 4 Chloride ions. The dipoles at opposite sides will cancel out (top to bottom & left and right), thus the shape will have 4 equally angled bonds by VSEPR theory and have a tetrahedral structure. CCl4 Molecular Geometry Shape and Bond Angles - YouTube A quick explanation of the molecular geometry of CCl4 including a description of the CCl4 bond anglesLooking at the CCl4 Lewis structure. This results in the cancellation of all polar vectors and a net zero dipole moment.
A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc... Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable. For the Lewis structure of CCl4 first, let's calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. 1) Draw the Lewis Structure of CCl4. What is the electron geometry of the central C atom? What is the molecular geometry of the central C atom? What is the hybridization of the central C atom? What is the molecular geometry or shape of the molecule? Does CCl4 have a dipole moment? 2) Draw the Lewis Structure of H2S. In the CCl4 Lewis Structure diagram, we always begin by introducing valence electrons from the central carbon atom. As a result, wrap around the central carbon atom’s bond pair valence electrons first. Carbon requires 8 electrons in its outermost valence shell to complete its octet.
Carbon tetrachloride | CCl4 - PubChem compound Summary Carbon tetrachloride Contents 1 Structures 2 Names and Identifiers 3 Chemical and Physical Properties 4 Spectral Information 5 Related Records 6 Chemical Vendors 7 Drug and Medication Information 8 Food Additives and Ingredients 9 Agrochemical Information 10 Pharmacology and Biochemistry
A CCL4 Lewis structure is a diagram that represents the electronic configuration of compounds.Lewis tied covalently structures are designed to provide a display of the atomic structure and the distribution of electrons in a given chemical compound.
Overview:CCl4 electron and molecular geometry. According to the VSEPR theory, C Cl4 possesses a tetrahedral molecular geometry and a C Cl4-like electron geometry. Because the centre atom, carbon, has four C- Cl bonds with the four Chlorine atoms surrounding it. The Cl-C- Cl bond generates a 109-degree angle in a tetrahedral geometry.
Here are the steps that I follow when drawing a Lewis structure. 1. Decide which atom is the central atom in the structure. That will be the least electronegative atom ( C ). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a C atom with four Cl atoms attached to it. 3.
Let's do the Lewis structure for CCl4, Carbon Tetrachloride, sometimes just called Carbon Tet. We'll start by looking at the valence electrons. Carbon is in group 4 or 14, so it has 4. Chlorine has 7 valence electrons, but we have 4 Chlorines so let's multiply that by 4. Four plus 28 equals 32 total valence electrons to work with.
Follow these steps. Step 1 Count the total number of valence electrons in the molecule. As an example, let's use carbon tetrachloride, CCl4. The single carbon atom contains four valence electrons, and each of the four chlorine atoms contains seven valence electrons. Therefore, the number of valence electrons for this molecule is 4 + (4 7) = 32.
The Lewis diagram for CCI4 is: The electron-pair geometry around the C atom in CCL4 is There are lone pair(s) around the central atom, so the geometry of CCl4 is H-C-H B. The Lewis diagram for CH is: The electron-pair geometry around the C atom in CH is There arelone pair(s) around the central atom, so the geometry of CH is
A CCL4 Lewis structure is a diagram that represents the electron setup of covalently bonded substances. Therefore, a carbon atom will share each of its 4 external electrons with a single chlorine atom, offering the single carbon atoms and 4 chlorine atoms a complete external shell of electrons. … Does CCl4 follow the octet guideline?
The Lewis diagram for CC4 ::Cl-c-Ci: :CI: The electron-pair geometry around the C atom in CCl4 is There arelone pair(s) around the central atom, so the geometry of CCl4 is CI: B. The Lewis diagramh for AICI3 is: AI The electron-pair geometry around the Al atom in AICl, is There arelone pair(s) around the central atom, so the geometry of AICl, is
I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over hybridization, shape and bond angle.
Nov 12, 2018 · A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.


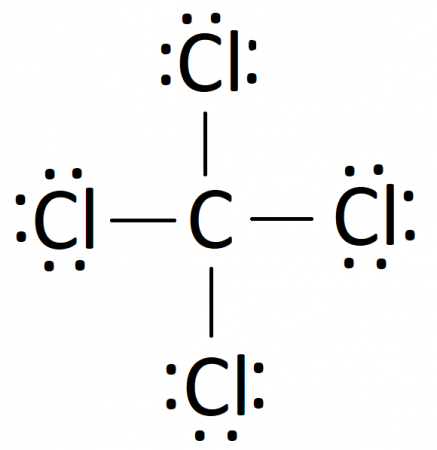



















0 Response to "41 lewis diagram for ccl4"
Post a Comment