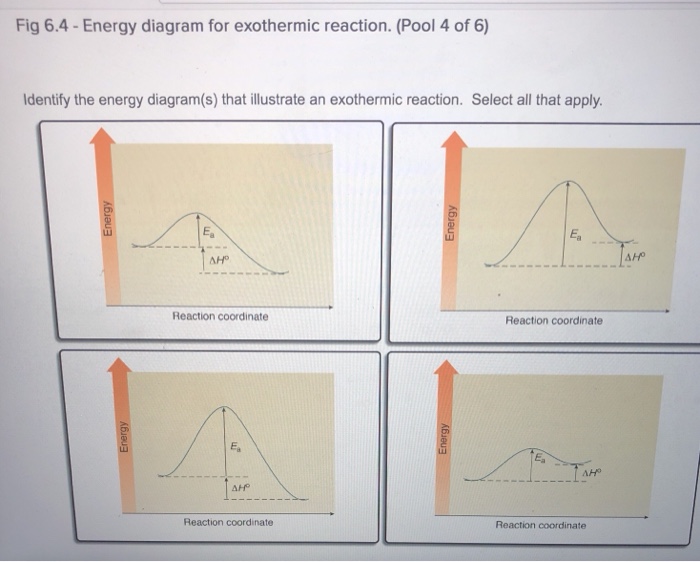
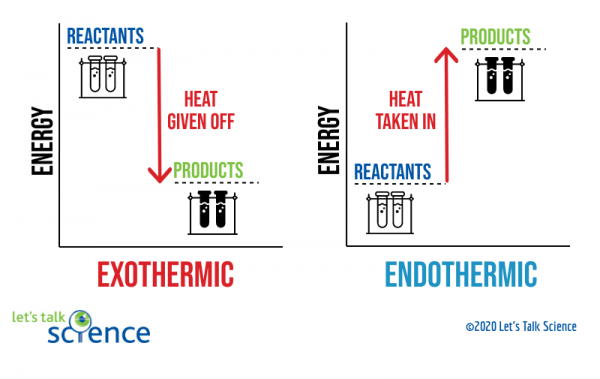
42 how will an energy diagram look for an exothermic reaction?
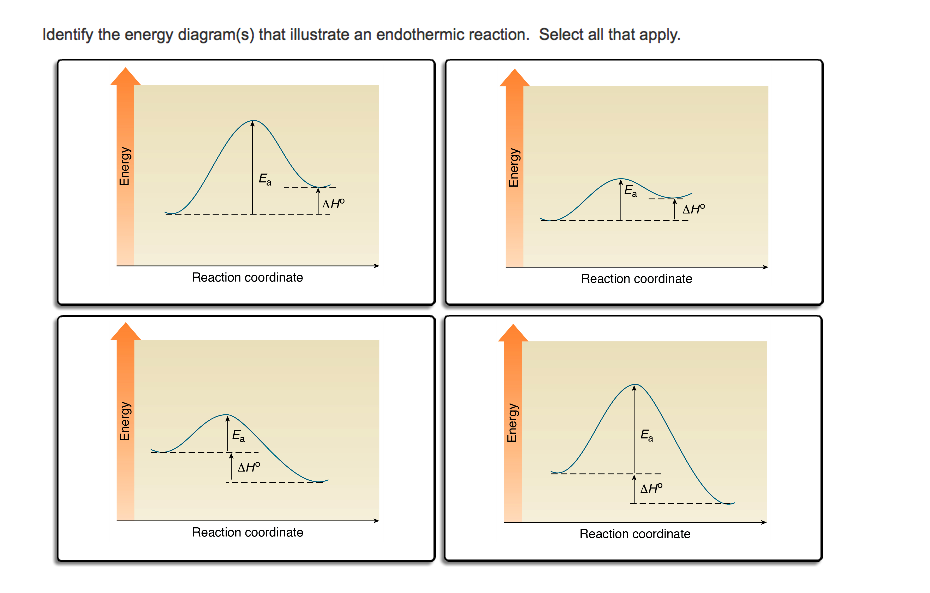
A complete enthalpy diagram will include starting energy, ending energy, and E a and delta H. This enthalpy diagram has starting products, ending products, delta H, and activation energy labeled... Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
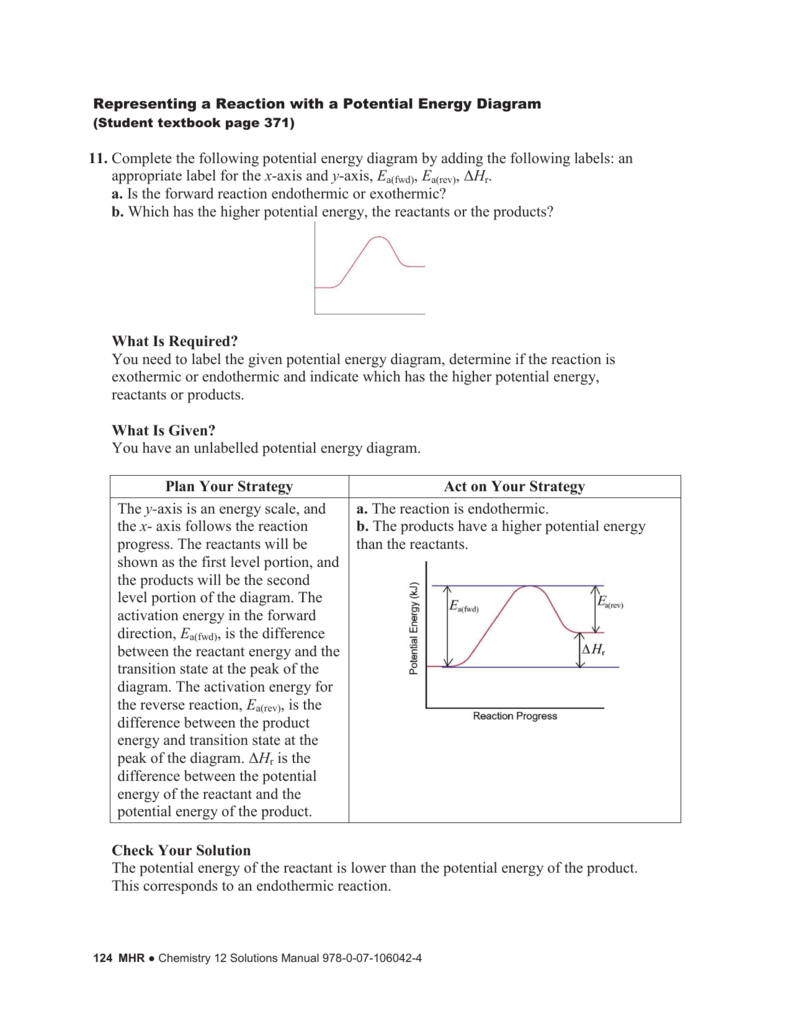
What does an enthalpy profile diagram for an exothermic reaction look like, including labels for enthalpy change and activation energy? [diagram] Taken on it own, is the breaking of bonds an endothermic or exothermic process?

How will an energy diagram look for an exothermic reaction?
b) an endothermic reaction. Diagram 1 c) the activation energy (E a ) is greater than the energy released (∆H) Diagram 3 d) a spontaneous exothermic reaction. Diagram 2 depending on the values but looks quite small. To be spontaneous needs to be ≤ 20kJ 2. How will an energy diagram look for an exothermic reaction? Chemistry. Answer Comment. 2 answers: Anuta_ua [19.1K] 5 months ago. 7 0. B.) The reactants are at a higher potential energy compared to the products. My reason being is because the reactants tend to gather "hyper" energy which is also known as potential energy and this will occur as shown in the answer as … 27.01.2022 · Energy Level Diagram For An Exothermic Reaction . Exothermic Reaction Uncatalyzed Exothermic Reaction And Catalyzed Exothermic Reaction Teaching Chemistry Study Chemistry Physical Chemistry . How Enzyme Reduce Activation Energy Enzymes Biology Biology Notes Biochemistry . Van T Hoff Equation In 2021 Chemical Equation Physical …
How will an energy diagram look for an exothermic reaction?. Answers: 2 to question: How will an energy diagram look for an exothermic reaction?
the reactants and products will be at the same potential energy.
the reactants are at a higher potential energy compared to the products.
the reactants are at a lower potential energy compared to the products.
there will not be an activation energy barrier. How will an energy diagram look for an exothermic reaction? The reactants and products will be at the same potential energy. The reactants are at a higher potential energy compared to the products. The reactants are at a lower potential energy compared to the products. There will not be an activation energy barrier. 2 See answers Answer 4.5 /5 21 HELP ASAP I WILL GIVE BRAINLIST The potential energy diagram for a reaction starts at 380 kJ and ends at 100 k… Get the answers you need, now! ... Exothermic reaction ... I have posted some more questions can you help me please with them. i can try :) let me take a look at them Advertisement Advertisement New questions in Biology. An exothermic reaction is a reaction in which energy is given off, or, in other words, a reaction that has a ΔH<0 (see: enthalpy).
16.01.2022 · In this video i go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. Shows whether a reaction is exothermic. Potential Energy Diagram For An Exothermic Reaction Cbsquared Gamsat Chemistry Resources Cb2gamsat Gamsat Potential Energy Chemistry Chemistry … Energy Diagrams Head back to the helpdesk Find more tutorials Try some practice worksheets . In this Helpdesk article, you'll learn everything you ever wanted to know (and more!) about energy diagrams. Here's what a sample energy diagram looks like (this is for an exothermic reaction): Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. (b) the energy difference between the reactants and the products, ΔH, heat of reaction Reaction profiles An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them....
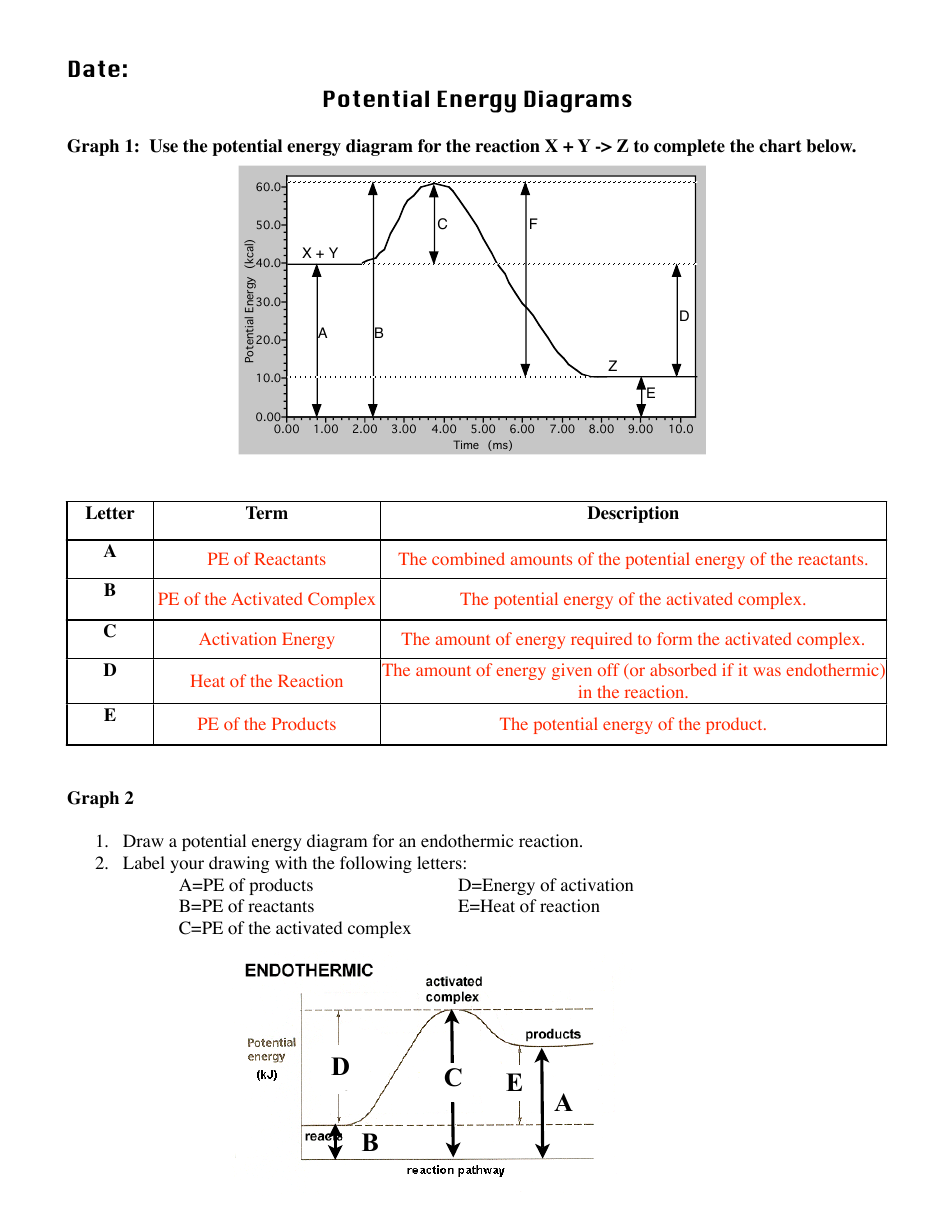
The activation energy for the reverse reaction is represented by A) 1 and 3 B) 3 and 4 C)2 and 3 D) 1 and 2 12.The potential energy diagram below shows the reaction X + Y Z. When a catalyst is added to the reaction, it will change the value of A) A B)B C) C D) D 13.In the potential energy diagram below, which letter 9. Jul 01, 2020 · Exothermic reactions release heat to the surroundings. Here’s the energy diagram for an exothermic reaction: Notice, the location of the REACTANTS (R). The diagram shows the reactant molecules with MORE energy than the product molecules (P). That means the product molecules have LESS energy than the reactants. Nov 23, 2021 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings. 21.09.2019 · Answers: 2 on a question: How will an energy diagram look for an exothermic reaction? the reactants and products will be at the same potential energy. the reactants are at a higher potential energy compared to the products. the reactants are at a lower potential energy compared to the products. there will not be an activation energy barrier.
Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
the diagrams graphically compare reactant energy to product energy as reaction occurs if reactants have more energy then products, the reaction is exothermic (loss of energy) if reactants have less energy then products, the reaction is endothermic (gain of energy)
Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ...
Have students look at the energy diagrams and explain how reactions can be exothermic or endothermic. Tell students that the word " exothermic" describes processes that give off heat. "Ex" indicates . out of . or . giving off. For example, exit, exhale (to breathe out), and exodus. "Thermic" indicates heat. " Endothermic"
substances. In an exothermic reaction enthalpy decreases when chemical energy is turned into heat energy and given out. So ΔT increases, and has a Q positive sign. This shows that ΔH has an opposite sign to Q and ΔT . Chemical energy Reaction Heat energy Chemical energy Heat energy ΔH (negative) ΔT (positive)
Energy diagrams between exothermic and endothermic reactions are slightly different, ... But, if you need a visual, look at our image description too.
Oct 24, 2021 · The green arrow is longer. Energy Diagram for Exothermic Reaction. Thus in an exothermic reaction energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small.
Many chemical reactions release energy in the form of heat, light, or sound. These are exothermic reactions. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (ΔS > 0) of the system. They are denoted by a negative heat flow (heat is lost to the surroundings) and decrease in enthalpy (ΔH < 0).
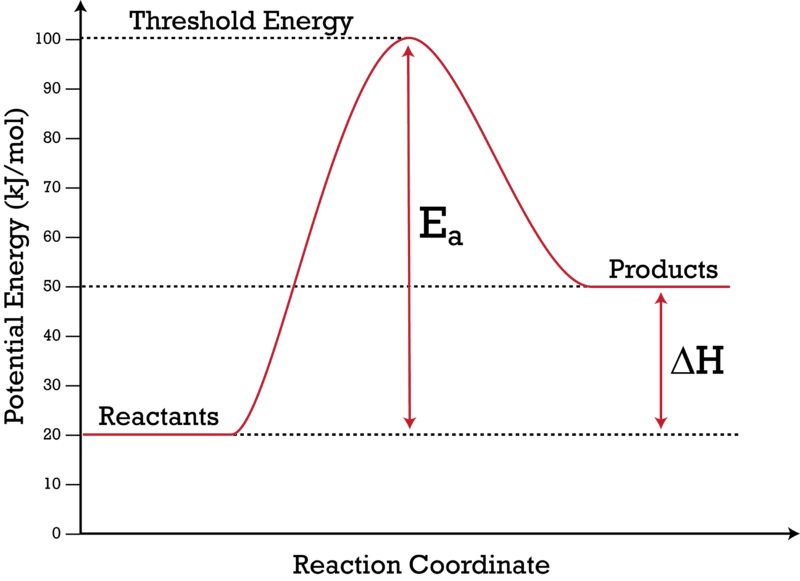
You can start with a generic potential energy diagram for an exothermic reaction. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.
This video illustrates how the potential energy of a chemical reaction changes during the reaction
So, here's how a rough sketch of a potential energy diagram would look like for this reaction. The difference between the energy level of the products and the energy level of the reactants is equal to the enthalpy change of reaction, #DeltaH_"rxn"#. Since the products are lower in energy, #DeltaH_"rxn"# carries a negative sign.
The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Calculate the energy of bonds breaking, bb. 2. Calculate the energy of bonds forming, bf. 3. Calculate the overall change, bb-bf. 4. Construct an energy level diagram. What are bond energies used for? They are used to calculate the overall energy change in a chemical reaction and it determines if the reaction is endo or exothermic.
During an exothermic reaction bonds break and new bonds form and protons and electrons go from a structure of higher potential energy to lower potential energy. During this change, potential energy is converted to kinetic energy, which is the heat released in reactions. In an endothermic reaction the opposite occurs.
The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
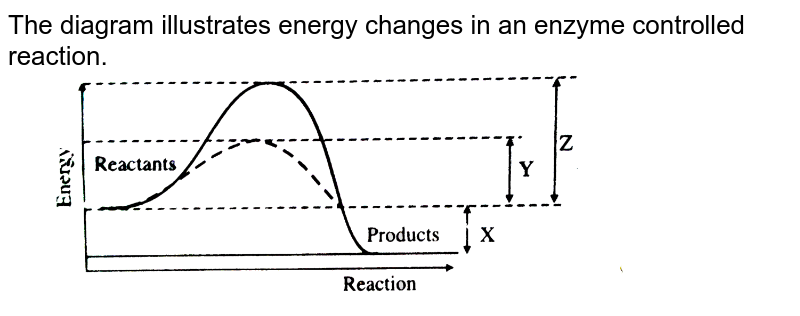
Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products.
Look at the reaction 2H 2 + O 2 2H 2O H = -483.6 kJ This equation tells us energy is released to surroundings therefore, exothermic reaction. In an energy diagram, the reactants have a higher energy than products. For the reverse reaction. 2H 2O 2H 2 + O 2 H = +483.6 kJ
Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Energy level diagrams Energy level diagrams are used to model energy changes during reactions. They show the relative energy levels of the products and reactants. Exothermic reaction The energy...
29.04.2014 · Moving from left to right, the curve will end up lower than where it started. This is because in an exothermic reaction, the potential energy of the products is lower than potential energy of the ...
27.01.2022 · Energy Level Diagram For An Exothermic Reaction . Exothermic Reaction Uncatalyzed Exothermic Reaction And Catalyzed Exothermic Reaction Teaching Chemistry Study Chemistry Physical Chemistry . How Enzyme Reduce Activation Energy Enzymes Biology Biology Notes Biochemistry . Van T Hoff Equation In 2021 Chemical Equation Physical …
How will an energy diagram look for an exothermic reaction? Chemistry. Answer Comment. 2 answers: Anuta_ua [19.1K] 5 months ago. 7 0. B.) The reactants are at a higher potential energy compared to the products. My reason being is because the reactants tend to gather "hyper" energy which is also known as potential energy and this will occur as shown in the answer as …
b) an endothermic reaction. Diagram 1 c) the activation energy (E a ) is greater than the energy released (∆H) Diagram 3 d) a spontaneous exothermic reaction. Diagram 2 depending on the values but looks quite small. To be spontaneous needs to be ≤ 20kJ 2.




























0 Response to "42 how will an energy diagram look for an exothermic reaction?"
Post a Comment