38 ammonia electron dot diagram
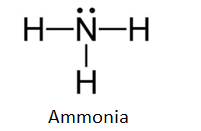
Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. The Lewis dot structure of ammonia, NH3, reveals that it ... The Lewis dot structure of ammonia, NH3, reveals that it has one lone pair of electrons and three bonds (each to a hydrogen) around the central nitrogen atom. According to VSEPR theory, what molecular shape will it have? i thought it was tetrahedral but . chemistry. Draw the Lewis structure of NO2- Assign formal charges to each atom in the O3 ...
Electron Dot Diagram Of Ammonium Ion Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th ...

Ammonia electron dot diagram
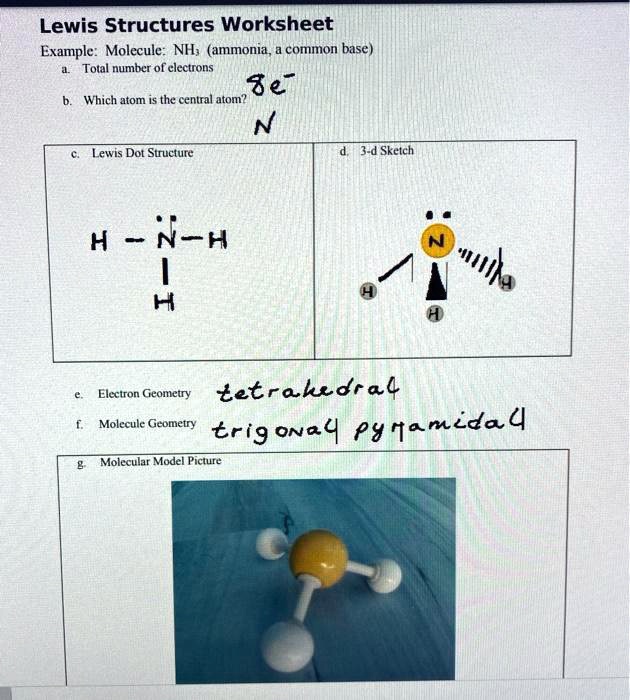
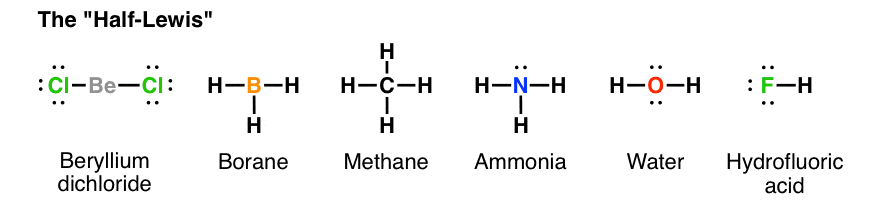
Lewis Structure for NH3 (Ammonia) - UMD Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer.It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.. Video: Drawing the Lewis Structure for NH 3 Draw an electron dot diagram to show the formation class ... Under normal conditions, both ammonia and ammonium ions will be present in normal water. -Electron dot diagrams can show how the electrons are involved in a bond formation. It is a diagram in which valence electrons of an atom are shown as dots and how they are distributed around the element's symbol. Electron Dot Diagram Of Ammonium Ion - schematron.org and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a positive ion and allowing it to form a dative bond with the Nitrogen. Meaning the nitrogen.
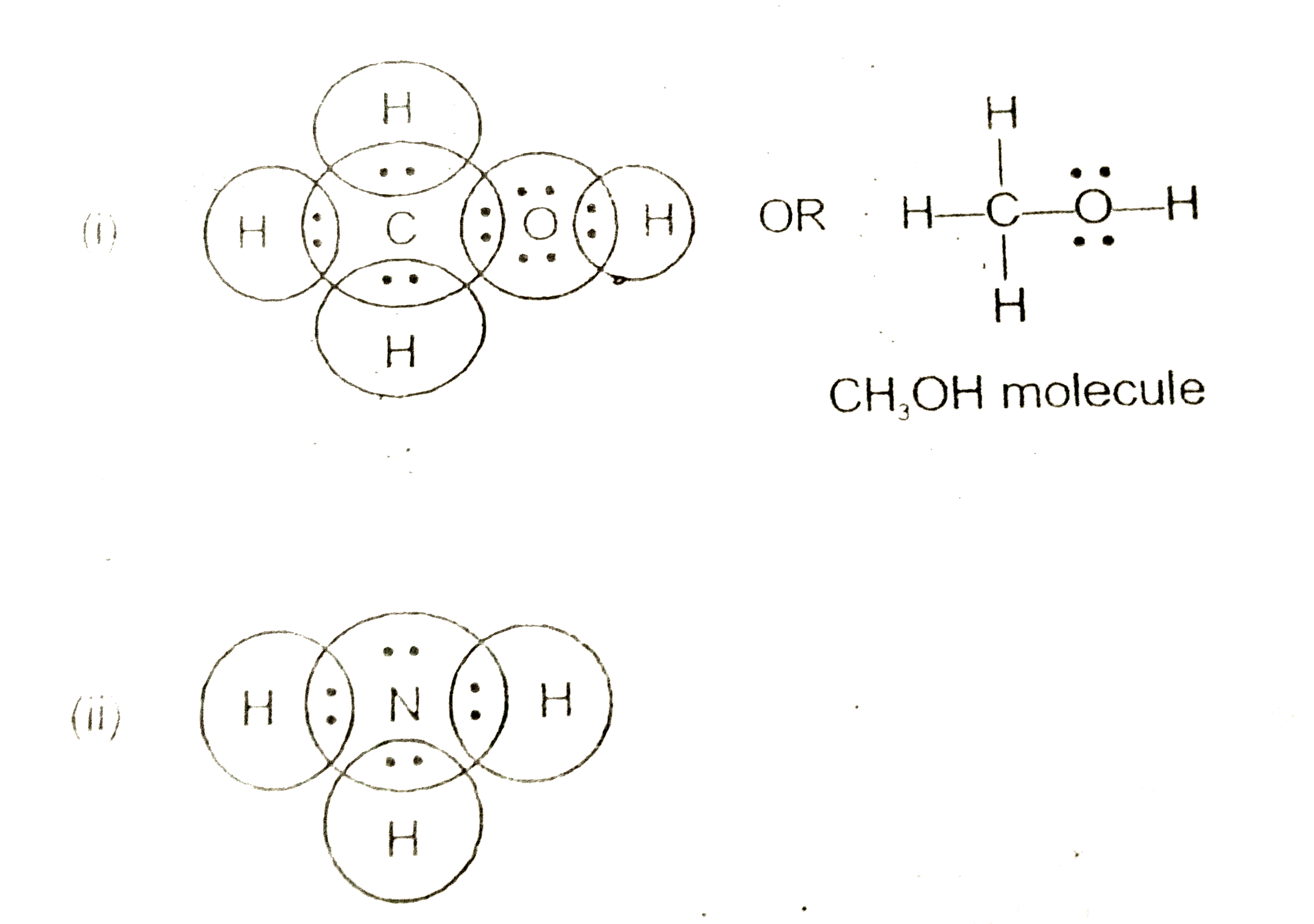
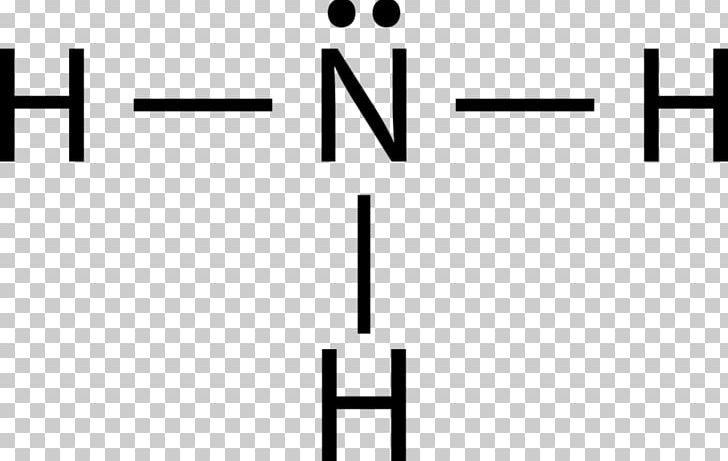
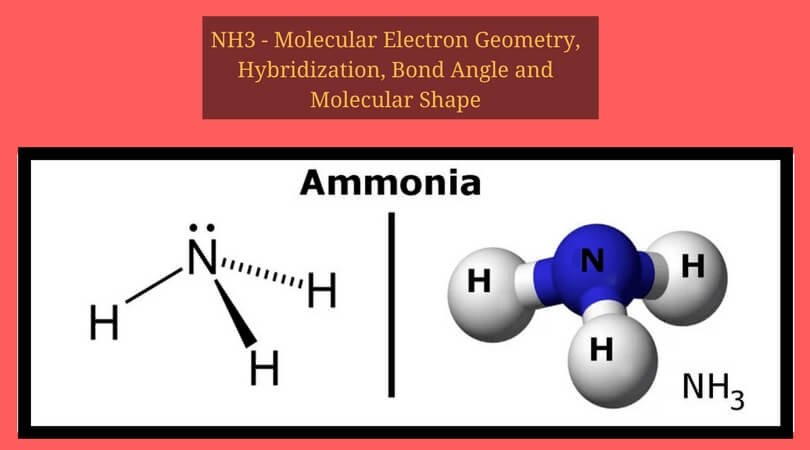
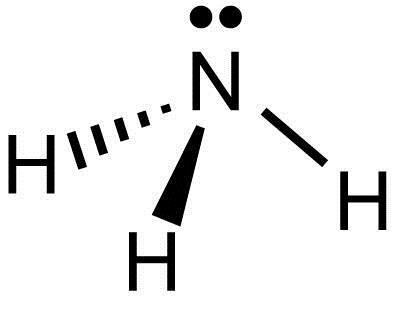
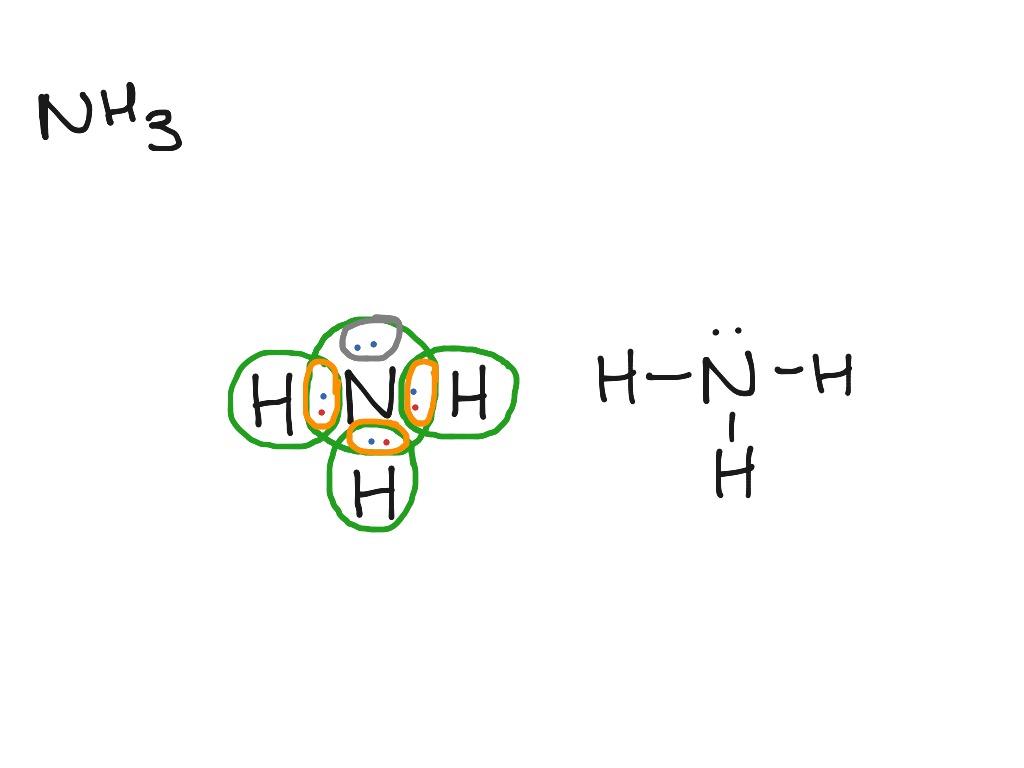
Ammonia electron dot diagram. GCSE CHEMISTRY - Covalent Bonding in an Ammonia Molecule ... This is a picture of an ammonia molecule. By sharing the two electrons where the shells touch each hydrogen atom can count 2 electrons in its outer shell and the nitrogen atom can count 8 electrons in its outer shell. These full outer shells with their shared electrons are now stable , and the NH 3 molecule will NH3 Lewis Structure, Geometry, and Hybridization ... It might surprise you that the ideal bond angle for the bent geometrical diagram is 109.5°. The molecular geometry of ammonia (NH3) is trigonal pyramidal or a distorted tetrahedral. It is because of the presence of a single lone pair of electrons on the nitrogen atom which is non-bonding in nature and exerts repulsion on the bonding orbitals. Lewis Structure Of Nh3 - ViralListClub.com Electron Dot Structure of NH3 by Jeff Bradbury - February 17 - Lewis Electron Dot Structure for ammonia molecule NH3. There is also a lone pair of electrons unbonded electrons on N. Drawing the Lewis Structure for NH 3 Ammmonia Ammonia NH 3 is a commonly tested Lewis structure due to its widespread use in agriculture as a fertilizer. Ammonia (NH3) Lewis Structure - Steps of Drawing NH 3 lewis structure. In the lewis structure of NH 3, there are three N-H bonds and one lone pair on nitrogen atom.There are no lone pairs on hydrogen atoms which cannot keep more than two electrons. Steps of drawing lewis structure of NH 3. You have to follow several steps to draw the lewis structure of NH 3.But, because ammonia is a simple molecule, these steps are not complex and do not ...
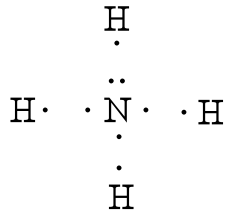
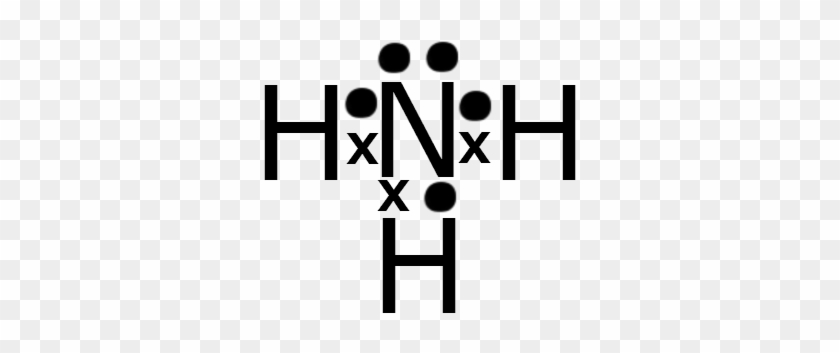
[Best Answer] draw the electron dot structure of ammonia ... THE ELECTRON DOT STRUCTURE OF AMMONIA IS SHOWN IN THE PICTURE. Explanation: The number of valence electron in nitrogen atom are 5. The valence electron in hydrogen atom is 1. There is a shortage of 3 electrons in nitrogen to complete its octet. So, it will form covalent bonds with 3 hydrogen atoms by sharing of electrons and ammonia molecule ... Nh3 Lewis Dot Structure - ViralListClub.com Nh3 lewis dot structure. The Lewis Dot Structure for NH3 Ammonia is shown above. Formed when the atoms need to form an octet but both. NH 3 Ammonia is a commonly tested Lewis structure. If the species is an ion add or subtract electrons corresponding to the charge of the ion. NH3 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale... Draw the electron dot stucture of ammonia molecule. - Toppr Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1]. Hard. View solution > Number of bonded pairs and lone pairs of electrons present in the central atom of ammonia molecule are. Medium. View solution > The molecule containing a triple covalent bond is :
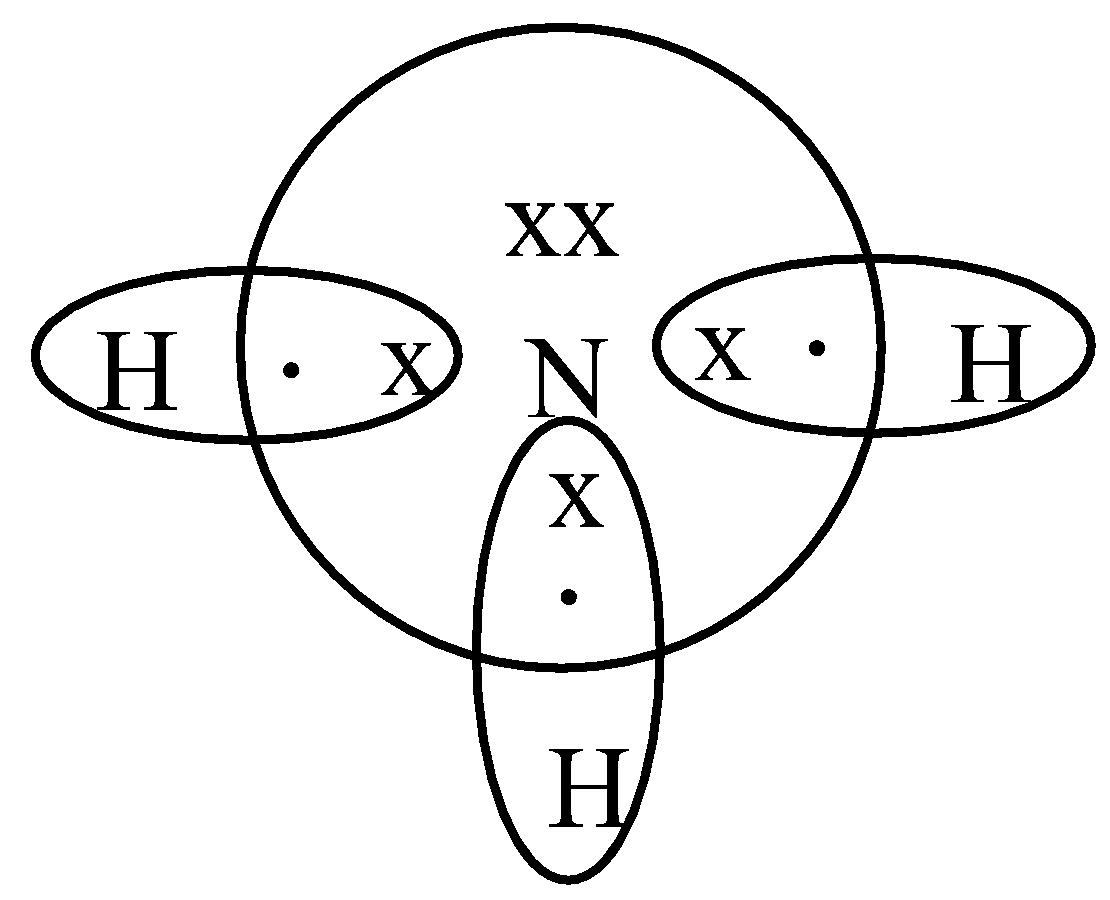
Draw A Lewis Dot Diagram Of Ammonia - Novocom.top lewis dot ammonia diagram structure electron ammonium structures molecule nh4 draw chemical nh3 example questions represented reactions ph3 showme interactive . dot ammonium electron diagram ammonia lewis structure ion nh4 wikipedia cation desk reference science . Electron dot structure of Ammonia | Carbon and its ... Electron dot structure of Ammonia || Carbon and its Compounds || class 10 || @10th science with VikashHello my online students,In this video, I have taught y... What is the electron dot structure for nh3? The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. One may also ask, what is the structure of ... Drawing dot- and- cross diagrams of Covalent Molecules - O ... Dot- and- cross diagram of covalent molecule ammonia (NH3) Let's look at drawing the dot-and-cross diagram of ammonia. Nitrogen is in group V of the periodic table. With 5 valence electrons, it needs 3 more electrons. So it will share 3 electrons to achieve stable octet configuration.
Electron Dot Diagram For Ammonia - the covalent bond ck 12 ... Here are a number of highest rated Electron Dot Diagram For Ammonia pictures upon internet. We identified it from obedient source. Its submitted by admin in the best field. We put up with this nice of Electron Dot Diagram For Ammonia graphic could possibly be the most trending topic in the manner of we portion it in google benefit or facebook.
What is the electron dot structure of ammonia? Lewis dot structure is defined as the structure which denotes the valence electrons around the elements. ... Its structure is tetrahedral. Ammonia is used in nitric acid production, as a fertilizer, and a cleaning solution. NH3, normally found as a gas, it is caustic and harmful in longterm exposure.
what is the electron dot structure for calcium ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. What is the electron dot structure of ammonia? -Ammonia consists of one nitrogen and three hydrogen.
By looking at the Lewis dot structure of ammonia NH3 class ... Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom. After the three valence electrons of Nitrogen have bonded with three Hydrogens, we still have two valence ...
NH3 Lewis Structure, Molecular Geometry, Hybridization ... Ammonia is a stable binary hydride having a Trigonal Pyramidal molecular geometry and sp3 hybridization. It has bond angles of 107 degrees and an electron geometry of tetrahedral. To know more about its polarity, read our blog on polarity. About Priyanka To read, write and know something new every day is the only way I see my day!
Electron Dot Structure of NH3 | Chemistry, Science | ShowMe Electron Dot Structure of NH3 by Jeff Bradbury - February 17, 2012 - Lewis Electron Dot Structure for ammonia molecule NH3
What is the Lewis dot structure for ammonia? - Colors ... This is the structure of ammonia or NH3. What is the use of Lewis dot structure? Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule.
Solved G. Derivatives of NH3 Assemble ammonia, NH3. Review ... G. Derivatives of NH3 Assemble ammonia, NH3. Review the electron dot diagram and the reason that this compound acts as a base (either Brønsted or Lewis). Replace 1, 2, 3 and finally 3, of the -H's on the N with-CH3 groups. You have just created primary, secondary and tertiary amines.
Lewis Dot Diagram Of Ammonia - schematron.org Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. ShowMe is an open learning community featuring interactive lessons on a variety of topics. The Lewis Dot Structure for NH4+ (Ammonium) is shown above.
Lewis Dot Structure Nh3 Covalent Bond - Novocom.top ammonia electron lewis lone dot diagram nitrogen pairs pair molecular geometry molecule formula atom ch4 electrons single chemistry bonds chemical . Liaison covalente de coordination â Wikipédia . ammonium lewis dot diagram ion electron ammonia structure bond covalent coordinate chemical hydrogen molecule .
Electron Dot Diagram Of Ammonium Ion - schematron.org and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a positive ion and allowing it to form a dative bond with the Nitrogen. Meaning the nitrogen.
Draw an electron dot diagram to show the formation class ... Under normal conditions, both ammonia and ammonium ions will be present in normal water. -Electron dot diagrams can show how the electrons are involved in a bond formation. It is a diagram in which valence electrons of an atom are shown as dots and how they are distributed around the element's symbol.
Lewis Structure for NH3 (Ammonia) - UMD Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer.It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.. Video: Drawing the Lewis Structure for NH 3





![Best Answer] draw the electron dot structure of ammonia ...](https://hi-static.z-dn.net/files/d15/527126fe03ca95d193acf105ed4e2f66.jpg)






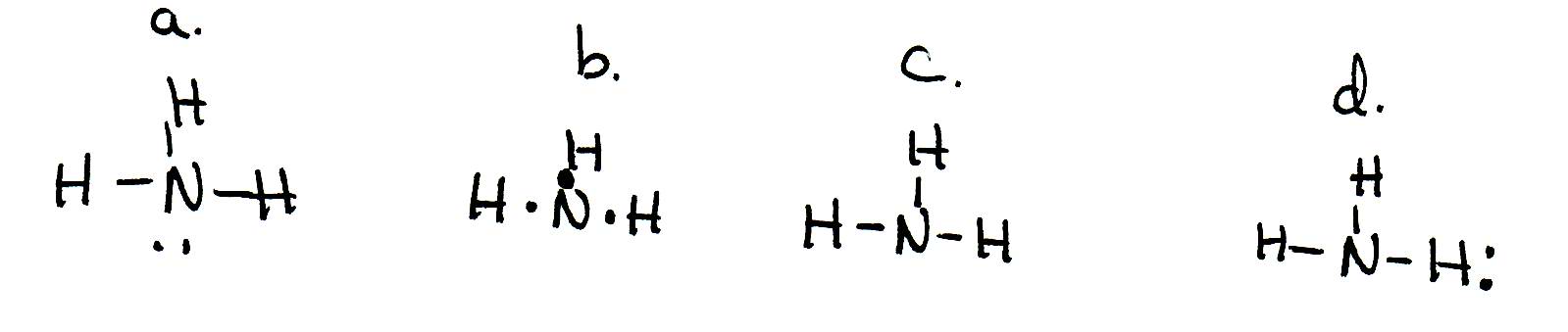




0 Response to "38 ammonia electron dot diagram"
Post a Comment