38 in an electron dot diagram of ethylene how many double bonds are present
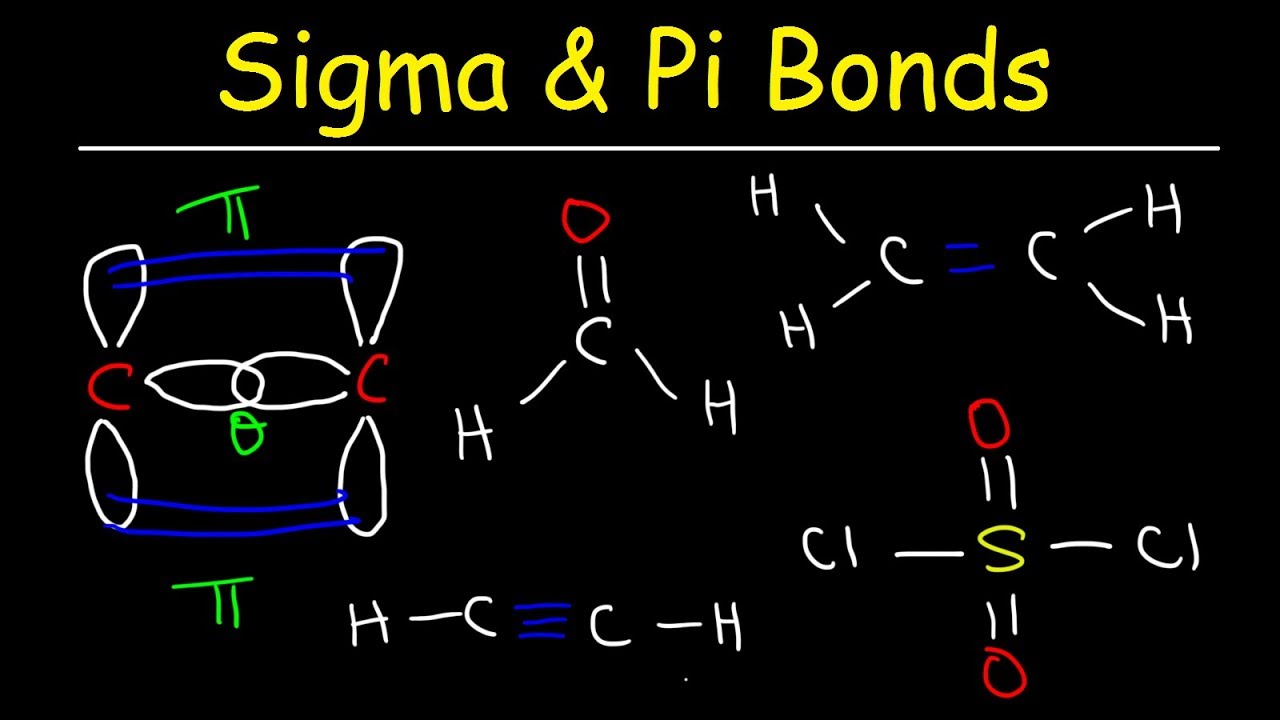
How many sigma and bonds are present in a molecule of ... The bond consists of two electron clouds which lie above and below the plane of carbon and hydrogen atoms. (a) Formation of ethylene (b) Molecular orbital structure molecule of ethylene Thus, ethylene molecule consists of four sigma C - H bonds, one sigma C - C bond and one bond between carbon-carbon atom. The bond length of carbon-carbon ... NOCl Lewis Structure, Molecular Geometry, Hybridization ... Lewis Structure of NOCl. Step 1: First take a molecule of NOCl. A nitrosyl chloride molecule consists of one atom of nitrogen, one atom of chlorine, and one atom of oxygen. Step 2: Now, we will find out the total number of valence electrons present in one NOCl molecule. To do so, have a look at the periodic table.
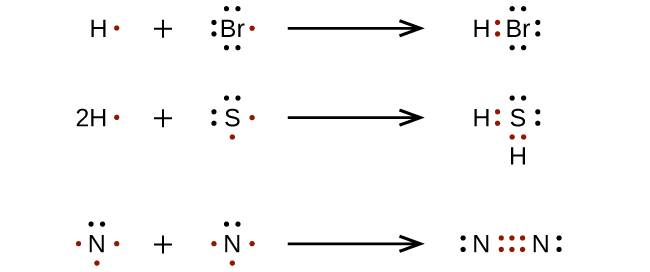
PDF 3.4 Covalent Bonds and Lewis Structures - Columbia University If an atom lacks an octet, use electron pairs on an adjacent atom to form a double or triple bond. • Example: All the atoms have octets in this Lewis structure. Table 1.4 How to Write Lewis Structures.... H C O N O H H..:..
In an electron dot diagram of ethylene how many double bonds are present
topblogtenz.com › ethene-c2h4-lewis-dot-structureC2H4 lewis structure, molecular geometry, bond angle ... C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. No lone pair is present on the central or outer atom in the lewis structure of C2H4. The lewis dot structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure. 1. Count total valence electron in C2H4 How do you know how many bonds are in a Lewis structure? The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus the number of valence electrons. This method works because each covalent bond that an atom forms adds another electron to an atoms valence shell without changing its charge. Draw Lewis structure of tetracyanoethylene and point class ... Draw Lewis structure of tetracyanoethylene and point the total number of sigma and pi bonds. Hint: Lewis structure shows a bonding between the atoms of a molecule. It is an electron dot structure that can be drawn for covalently bonded molecules as well as coordination compounds. It shows the position of an atom in different structures.
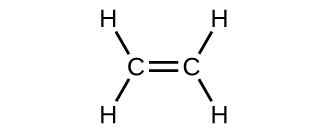
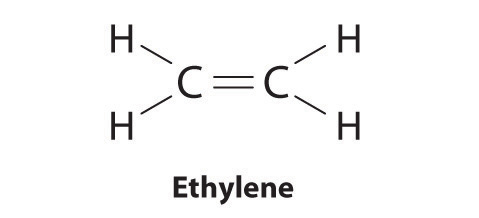
In an electron dot diagram of ethylene how many double bonds are present. In an electron dot diagram of ethylene (C2H4), how many ... Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms. The carbon atoms in ethene exhibit a valency of 4; therefore, in order to complete their outermost shell, the carbon atoms form a double bond with one another. Thus, the number of double bonds present in a molecule of ethylene is 1. Advertisement Answer 4.8 /5 20 Megadeth www2.chemistry.msu.edu › faculty › reuschPhotochemistry - Michigan State University In the case of the simple compound formaldehyde, the Lewis formula consists of two C–H sigma bonds, a C–O sigma bond, a C=O pi bond and two non-bonding electron pairs. As shown in the diagram on the right, two Lewis structures, differing in the hybridization of oxygen, may be drawn, The structure on the left is a common representation in ... Quick Answer: How Many Pairs Of Electrons Are Shared In ... Because ethylene is a double bond it is called an unsaturated hydrocarbon. Ethylene, C2H4, is a chemical compound composed of two carbon atoms and four hydrogen atoms. What do you call the bond having 2 shared electron? A Double bond is when two atoms share two pairs of electrons with each other. 4.17 Chemistry Unit Assessment K12 Flashcards - Quizlet In an electron dot diagram of ethylene (C2H4) how many double bonds are present?... What is the molecular shape of silicon tetrabromide? tetrahedron. Which of the following intermolecular forces plays a pivotal role in the unique properties of water? hydrogen-bonding.
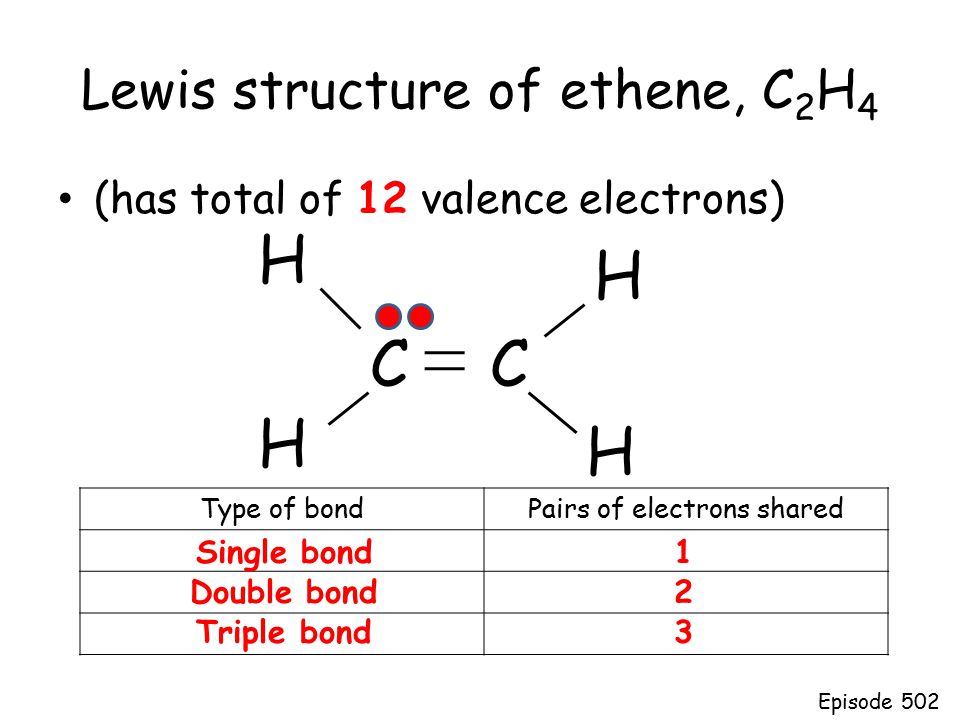
Chapter 8 - Chemical Bonds - CHE 105/110 - Introduction to ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... Double bonds or triple bonds between atoms may be necessary to properly ... how many electron pairs are shared between the carbon ... Formation of ethyne (C2 H2) molecule In ethyne each of two carbon atoms share one electron with a hydrogen atom and 3 electrons between themselves forming triple bond between the two carbon atom The Lewis dot structure is Lewis representation of. C2H4 Molecular Geometry / Shape and Bond Angles 4.17 Unit Test: Chemical Bonding - Part 1 v2 - Quizlet In an electron dot diagram of ethylene (C2H4) , how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron. Which intermolecular force plays a pivotal role in the unique properties of water? hydrogen bonding. Which element does not form a monatomic ion? The number of covalent bonds in ethylene is: - Toppr 1 double and 5 single bonds Medium Solution Verified by Toppr Correct option is D) Ethene means C2H4 and it is an alkene. The total number of covalent bonds in the molecule of ethene will be 6 ( one sigma bond and one pi bond between the two carbon atoms and 4 hydrogen atoms remain attached to the two carbon atoms by 4 sigma bonds ) CH 2 =CH 2
K12. 4.17 Unit Assessment: Chemical Bonding, Part 1. 2018 ... In an electron dot diagram of ethylene (C2H4), how many double bonds are present? One. What is the molecular shape of silicon tetrabromide? Tetrahedron. Which of the following intermolecular forces plays a pivotal role in biological molecules such as proteins and DNA? Hydrogen bonding. how many electron pairs are shared between the carbon ... And in ethylene there are 4 electrons shared between the carbon atoms; the remaining 2 electrons constitute half of the C−H bonds. How many bonds pairs of electrons are in ch2 ch2? So, there are one carbon carbon double bond and four carbon hydrogen single bonds. Hence, total number of bonds = total number of electron pairs = 2 + 4 = 6. en.wikipedia.org › wiki › MoleculeMolecule - Wikipedia The simplest of molecules is the hydrogen molecule-ion, H 2 +, and the simplest of all the chemical bonds is the one-electron bond. H 2 + is composed of two positively charged protons and one negatively charged electron , which means that the Schrödinger equation for the system can be solved more easily due to the lack of electron–electron ... What is the electron dot structure of ethene? - Quora 10 Jan 2018 — #Lewis structures extend the concept of the electron dot diagram by adding ... on those circles represent how many electrons are present within that level.2 answers · 3 votes: Thank youHow many sigma bonds and pi bonds are present in ...7 answers1 Feb 2018How many electron pairs are shared between carbon ...11 answers8 Sept 2020How many double bonds are in CO2? - Quora8 answers11 Jan 2021What is the electron dot structure of ethane? - Quora3 answers29 Jul 2018More results from
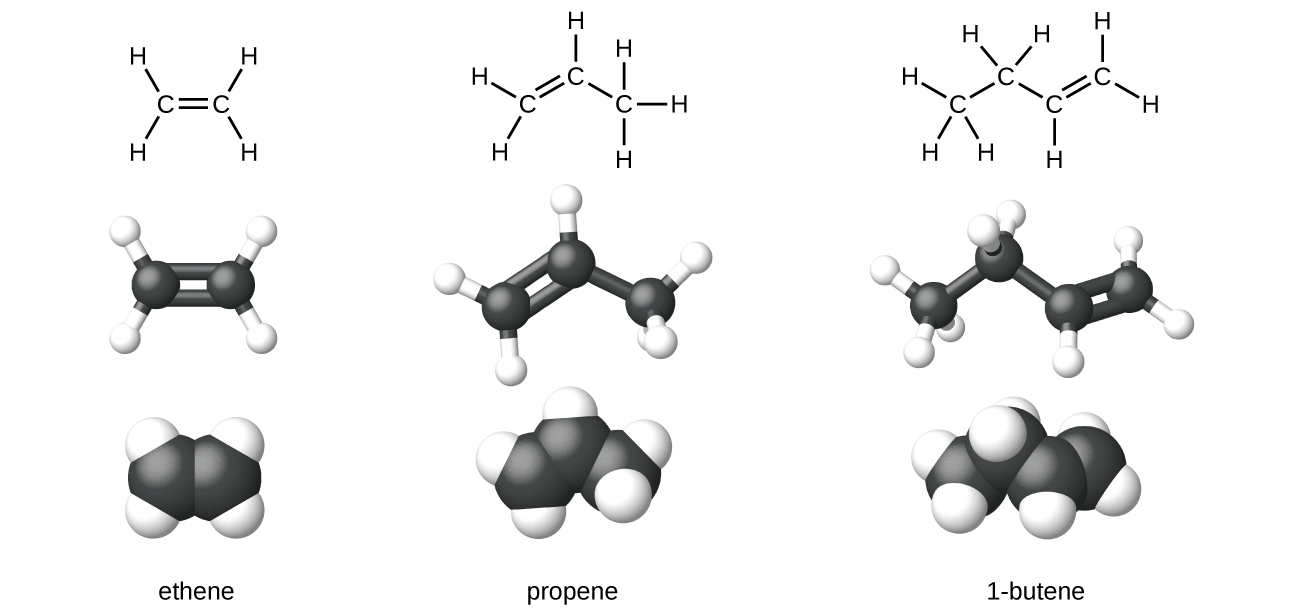
Sigma and Pi Bonds | Chemistry for Non-Majors Ethene (C 2 H 4) contains a double covalent bond between the two carbon atoms and single bonds between the carbon atoms and the hydrogen atoms. The entire molecule is planar. Figure 1. Geometry of ethene molecule. As can be seen in the figure above, the electron domain geometry around each carbon independently is trigonal planar.
4.16 Unit Test: Chemical Bonding Flashcards | Quizlet Z+. Which property is a characteristic of an ionic compound? high melting point. Hydrogen bonds can be found between molecules of which substance? NH3. Which compound contains a triple bond? acetylene (C2H2) In an electron dot diagram of ethylene (C2H4), how many double bonds are present? one.
Chemistry 4.11 Mid-Unit Test Review: Chemical Bonding ... In an electron dot diagram of ethylene (C2H4) how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron. Which of the following intermolecular forces plays a pivotal role in the unique properties of water? OR
In an electron dot diagram of ethylene (c2h4), how many ... Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms. The carbon atoms in ethene exhibit a valency of 4; therefore, in order to complete their outermost shell, the carbon atoms form a double bond with one another. Thus, the number of double bonds present in a molecule of ethylene is 1. Answer from: Quest SHOW ANSWER
› revision-notes › cbse-class-11Class 11 Chemistry Revision Notes for Chapter 4 - VEDANTU Lewis symbols or electron dot symbols are the names given to these symbols. 4. Ionic Bond: Ionic bonding is a sort of chemical bonding that occurs when electrons are transferred from one atom or molecule to another. One atom loses an electron in this process, which is then gained by another atom.
how many c2h4 molecules are contained in 45.8 - Lisbdnet.com In C2H4, if we look into the lewis structure, we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. According to the VSEPR chart, the shape of the ethene molecule is trigonal planar. There are two triangles overlapping each other as we can see in the diagram. See also how far inland does a storm surge go
topblogtenz.com › propene-c3h6-lewis-structureC3H6 Lewis structure, Hybridization, Molecular geometry, Polarity Bonding electrons that take part in the formation of bonds and are represented as single, double, or triple bonds between the atoms in the lewis diagram. So, as per the C3H6 Lewis structure, there are 7 single bonds and one double bond are present means 9 bonded pair that contains 18 bonding electrons.
Covalent Bond - Definition, Types, Properties, and Examples In this case, a single bond is formed between hydrogen and chlorine by sharing one electron. Double Bonds. A double bond is formed when two pairs of electrons are shared between the two participating atoms. It is represented by two dashes (=). Double covalent bonds are much stronger than a single bond, but they are less stable.
which of the following ions will most likely ... - Brainly.com Ethylene has four single covalent bonds and one double covalent bond. The Lewis dot structure is used to show the bonding between the valence electrons of a compound. Ethylene has 12 valence electrons and these are shared in this manner: 4 [double bond], 2 [single bond], 2, 2, 2.
quizlet.com › 195082504 › chem-flash-cardschem Flashcards - Quizlet A. How many moles of Ba(OH)2 are present in 135 mL of 0.400 M Ba(OH)2? B. How many moles of HNO3 are present if 5.40×10−2 mol of Ba(OH)2 was needed to neutralize the acid solution? C. What is the concentration of HNO3 if 0.108 mol are present in 775 mL of the solution?
C2H2 (Acetylene | Ethyne) Lewis Structure C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
Ethylene | CH2=CH2 - PubChem The rate constant for the gas-phase reaction of ethylene with photochemically-produced hydroxyl radicals is 7.9X10-12 cu cm/molecule-sec at 25 °C (1). This corresponds to an atmospheric half-life of about 2 days at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm (2).
In an electron dot diagram of ethylene (c2h4), how many double ... Answer: 3 ✓ on a question ➜ In an electron dot diagram of ethylene (c2h4), how many double bonds are present? a. none b. one c. two d. three - the ...3 answers · 3 votes: Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms.The carbon atoms in eth...
Dot and cross diagrams - Covalent bonds - OCR 21C - GCSE ... The formula is H 2 O so the dot and cross diagram includes two H atoms and one O atom. H has 1 outer electron. O has 6 outer electrons. The H circles must each overlap the O circle. Question Draw ...
Write the electron dot structure of ethene molecule (C2H4) ... question ✍️ Write the electron dot structure of ethene molecule (C2H4) . ... Solve any question of Chemical Bonding and Molecular Structure with:-.1 answer · Top answer: Electron dot structure of C2H4 ⟶ H2C = CH2 .
Ethylene (C2H4) - Structure, Molecular Mass, Physical and ... Ethylene (C2H4) - Ethylene is an unsaturated organic compound with the chemical formula C2H4. Each carbon atom of ethylene is sp2 hybridization. Ethylene is used in the manufacturing of polyethylene. It is a colorless gas which has a sweet odor and taste. To learn more about the structure, Molecular Mass, Physical and Chemical Properties, Uses and FAQs of Ethylene, Visit BYJU'S for more content
techiescientist.com › c2h4-lewis-structureC2H4 Lewis Structure, Molecular Geometry ... - Techiescientist Mar 02, 2021 · Since there are two bonds forming here, we will have a double bond structure. Hence, C2H4 is an alkene. Here, we have got the most suitable and appropriate Lewis Structure Sketch of ethylene. Molecular Geometry. When we draw the Lewis Structure of C2H4, we find a linear 2-D representation. In reality, the molecular shape of ethene is not linear.
covalent bonding - double bonds - chemguide Two oxygen atoms can both achieve stable structures by sharing two pairs of electrons as in the diagram. The double bond is shown conventionally by two lines joining the atoms. Each line represents one pair of shared electrons. Carbon dioxide, CO2 Ethene, C2H4 Ethene has a double bond between the two carbon atoms.
Draw Lewis structure of tetracyanoethylene and point class ... Draw Lewis structure of tetracyanoethylene and point the total number of sigma and pi bonds. Hint: Lewis structure shows a bonding between the atoms of a molecule. It is an electron dot structure that can be drawn for covalently bonded molecules as well as coordination compounds. It shows the position of an atom in different structures.
How do you know how many bonds are in a Lewis structure? The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus the number of valence electrons. This method works because each covalent bond that an atom forms adds another electron to an atoms valence shell without changing its charge.
topblogtenz.com › ethene-c2h4-lewis-dot-structureC2H4 lewis structure, molecular geometry, bond angle ... C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. No lone pair is present on the central or outer atom in the lewis structure of C2H4. The lewis dot structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure. 1. Count total valence electron in C2H4
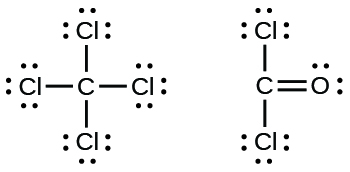







/ethylene-56a12bda5f9b58b7d0bcbafc.jpg)










0 Response to "38 in an electron dot diagram of ethylene how many double bonds are present"
Post a Comment