38 molecular orbital diagram c2
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms. Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+.
Investigation of bond orientational order of new Schiff ... DFT studies, antifungal activity and molecular docking studies, Journal of Molecular Structure., 1206 (2020), p. 127639 , 10.1016/j.molstruc.2019.127639 Article Download PDF View Record in Scopus Google Scholar

Molecular orbital diagram c2
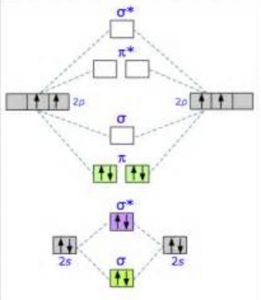
By molecular orbital theory, bond order of c2 is 2, while... - Quora This is how the Molecular Orbital diagram for C2 would look like. 2,Molecular Orbital Theory shows that there are two sets of paired electrons in a degenerate pi bonding set of orbitals. This gives a bond order of 2, meaning that there should exist a double bond between the two carbons in a C2... PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently lower in energy than the valence Does your MO diagram agree with this expectation? Determine the primary MOs that determine the bond order. Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Molecular orbital diagram c2. Chapter 1: Molecular Orbital Concepts Chapter 1: Molecular Orbital Concepts A. Concepts of MO Theory. 1. Strong Covalent Bonds. Consider the pi bond of ethene in simple molecular orbital terms (The qualitative results would be the same for any pi or sigma bond. Introduction to Molecular Orbital Theory This section illustrates pictorially molecular orbitals for several organic and inorganic molecules. If possible - the energy level diagram is included and clicking upon the relelvant level will generate the accompanying molecular orbital in the right-hand frame. Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Polyatomic Molecular Orbital Theory. Transformational properties of atomic orbitals. The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in Molecular Orbital Theory - BH3. BH3 has a C3 principal axis of symmetry, 3 C2 axes (┴ C3), 3 σv...
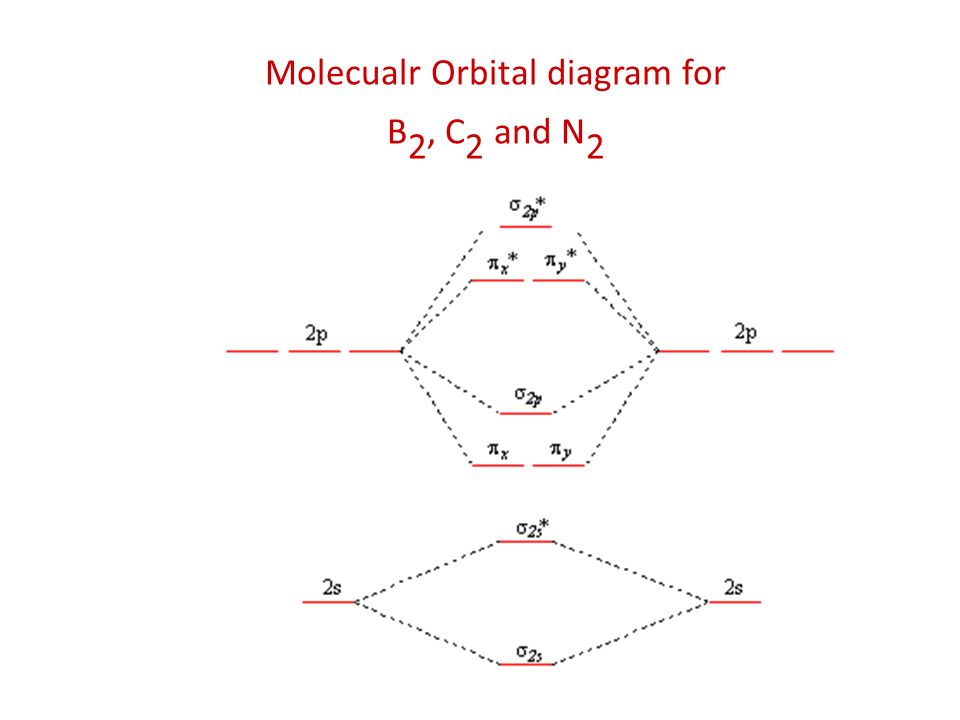
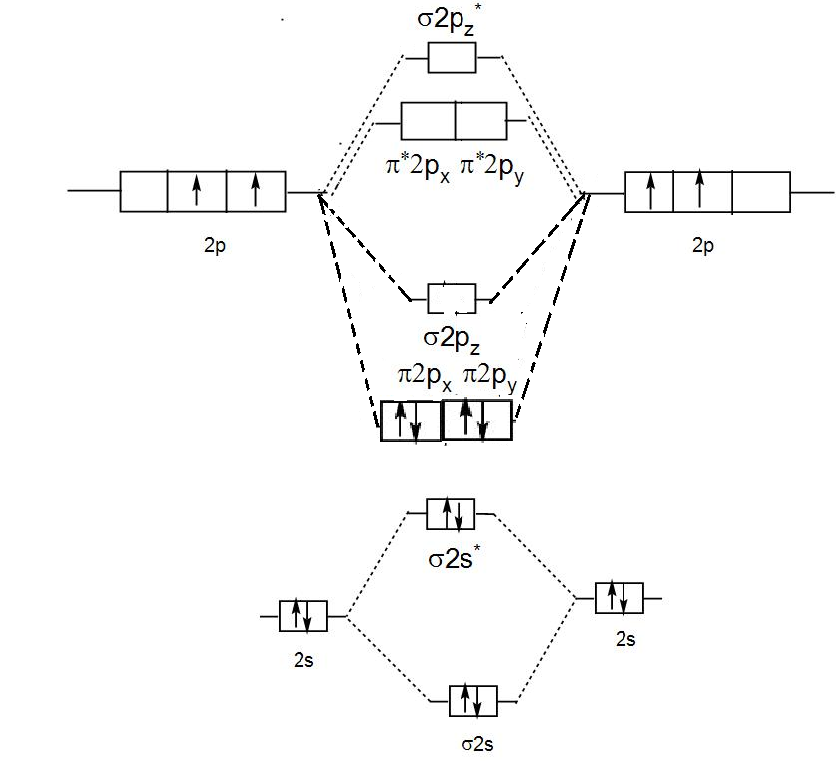
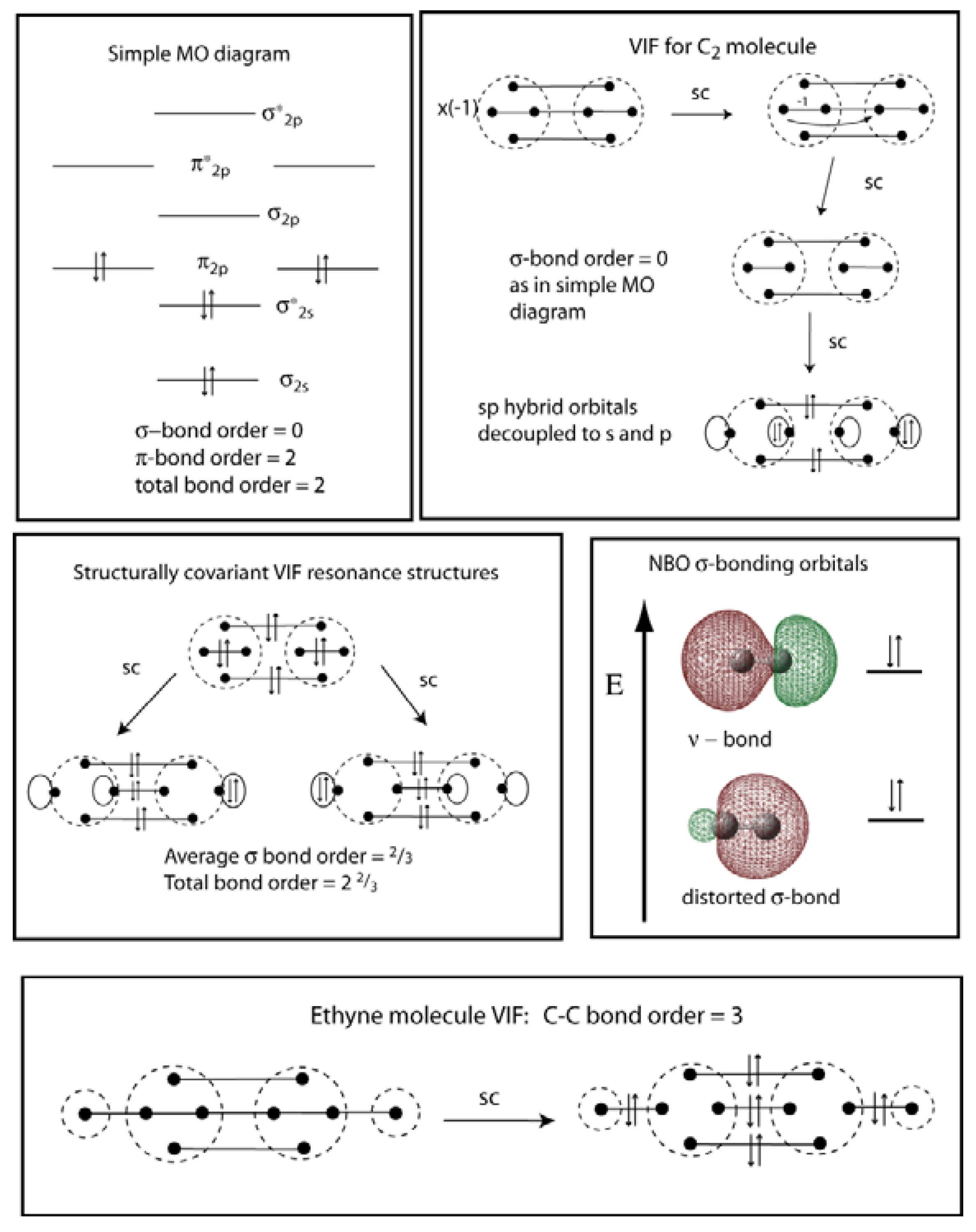
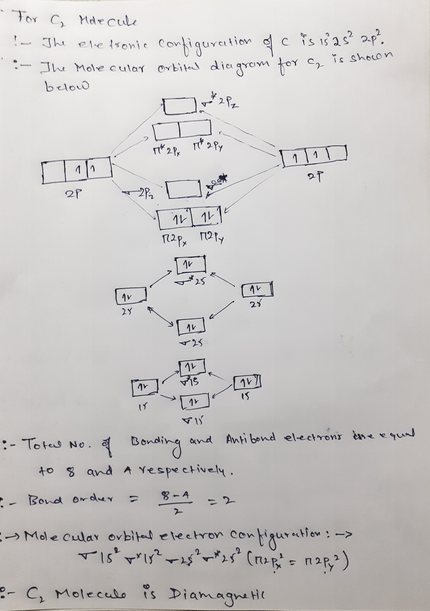
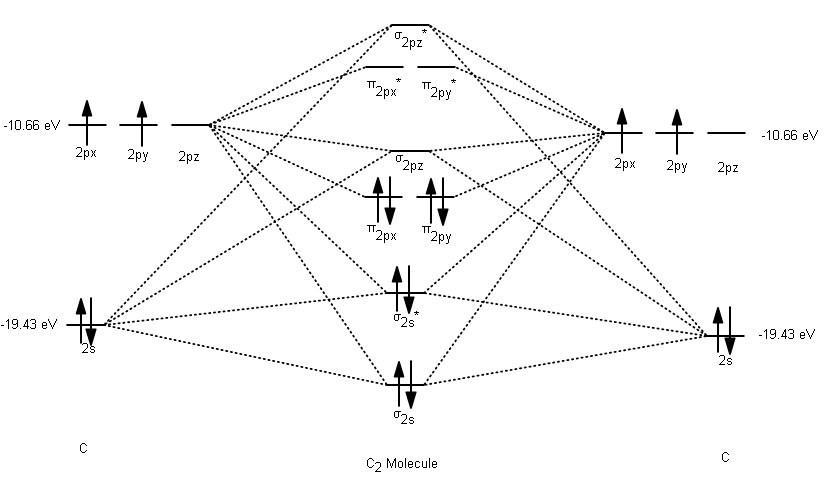
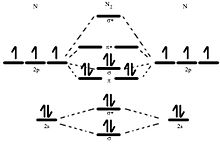
PDF Molecular | 90" (porbitals). This dilemma has been resolved by orbital MOLECULAR ORBITAL and valence bond calculations of the w-electron energies of unsaturated molecules custom-arily start with models in which appropriate atomic orbitals a r e assigned to each nucleus to provide a framework for -notions of the binding electrons. Atomic orbital r e p r e... Energy level diagram for Molecular orbitals - Chemical Bonding and... The molecule is diamagnetic. The double bond in C2 consist of both Pi bonds because the four electrons are present in the two pi molecular orbitals. Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e-. Reply. Mrs Shilpi Nagpal says. PDF Figure 9.32: The molecular orbital energy-level diagram for • The following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the... Molecular Orbital (MO) Diagram of C2 - YouTube Molecular Orbital Diagram for Carbon Dimer (C2).Fill from the bottom up, with 8 electrons total.Bonding Order is 2, and it is Diamagnetic.sigma2s(2),sigma2s...
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. For example, homonuclear diatomic molecules of second row elements like Li 2 , Be 2 , B 2 , C 2 , N 2 , the σ 2p z MOs is higher in energy than π 2px and π 2py MOs. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry source : isite.lps.org. Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals The solid lines represent the relative energies of the indicated atomic and molecular orbitals. (a) The diagram for H2, He2, Li2, Be2, B2, C2, and N2...
ʻOumuamua - Wikipedia ʻOumuamua is small and not very luminous. It was not seen in STEREO HI-1A observations near its perihelion on 9 September 2017, limiting its brightness to approximately 13.5 mag. By the end of October, ʻOumuamua had already faded to about apparent magnitude 23, and in mid-December 2017, it was too faint and fast moving to be studied by even the largest ground …
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8.34).
[Solved] The molecular orbital diagram for the carbide... | Course Hero Q: Use the molecular orbital diagram shown to determine which of the following is paramagnetic. None of the ions are para. Q: Can you answer the Twelve Chemical Reactions part 1-12 and Eleven Elegant Elements part 1-11 and the Ten Magnificent Mol.
8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
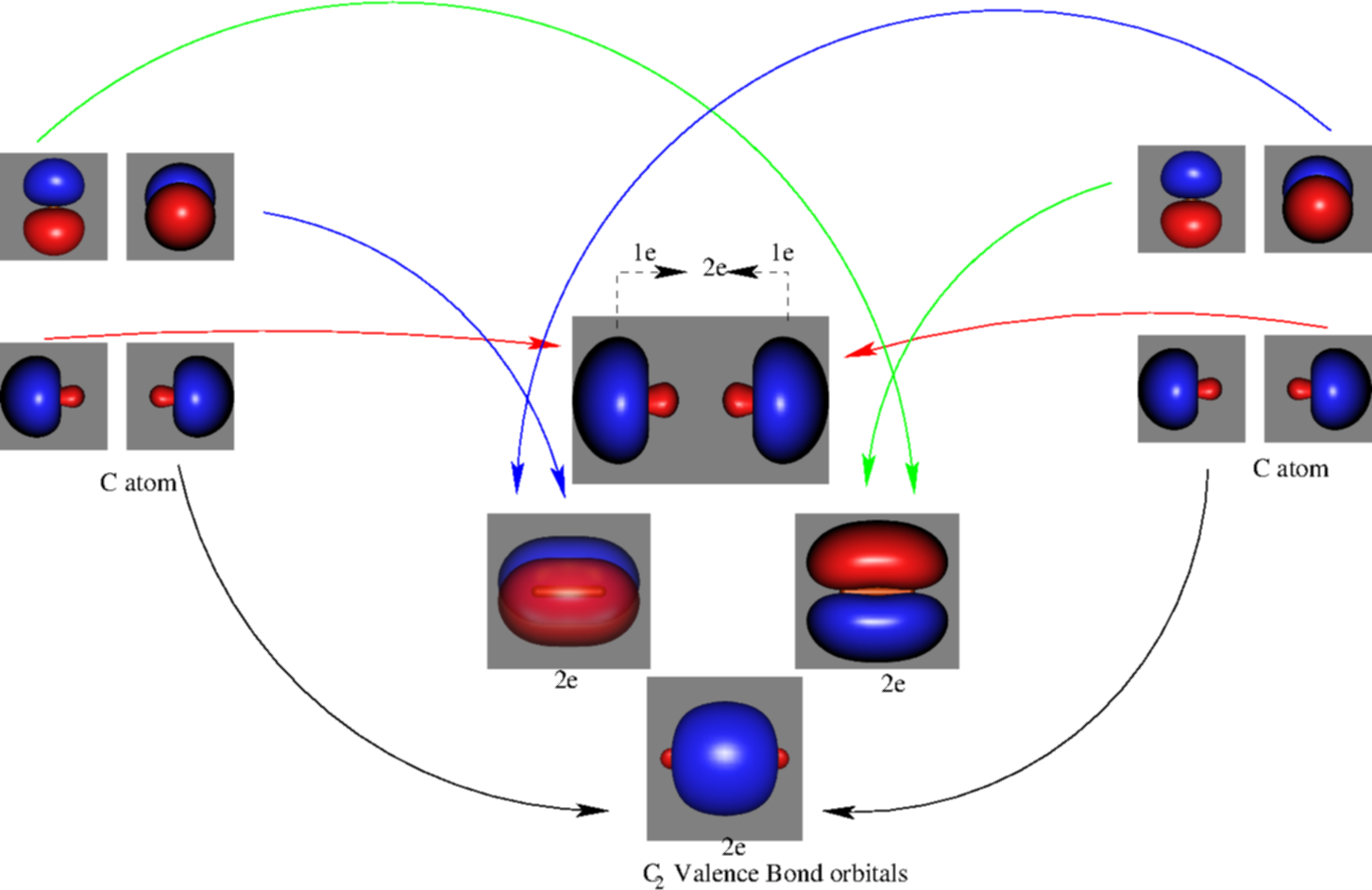
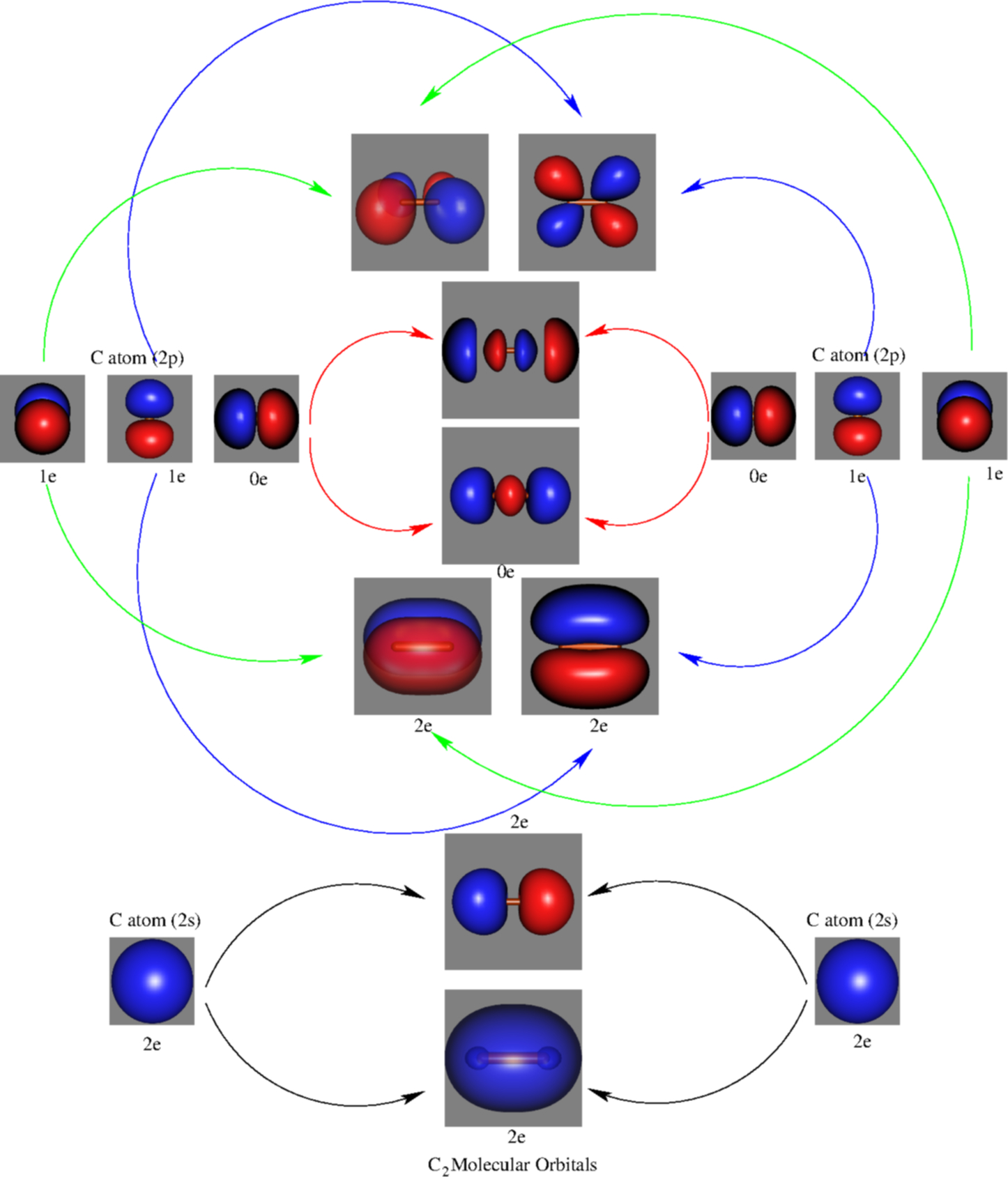
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
PDF Procedure for Constructing Molecular Orbital Diagrams Based on... Last time you learned how to construct molecule orbital diagrams for simple molecules based on the symmetry of the atomic orbitals. We could use the symmetry-based method to construct molecular orbital diagrams for larger molecules as well, but this can get complicated for larger structures.
molecular orbital diagram of C2 - - Brainly.in Molecular orbital diagram of C2 -. 2. See answers. Advertisement.
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2p_(sigma), so that is where the extra electron will be added. The problem provides you with the MO diagram for the #"C"_2# molecule, so all you really have to do here is add an electron to that diagram.
Pentane Formula: Formula & Structure, Properties - Embibe Pentane Formula: Pentane is the fifth homologue of the alkane series. It is a gasoline odour, easily liquefied gas with a chemical formula of \({{\rm{C}}_5}{{\rm{H}}_{12}}.\) It is typically used to create a polystyrene foam; a foam used to make insulation …
Asteroid - Wikipedia Asteroid discovery methods have dramatically improved over the past two centuries. In the last years of the 18th century, Baron Franz Xaver von Zach organized a group of 24 astronomers to search the sky for the missing planet predicted at about 2.8 AU from the Sun by the Titius-Bode law, partly because of the discovery, by Sir William Herschel in 1781, of the planet Uranus at …
Molecular Orbitals of The Allyl Cation, Allyl Radical, and ... The lowest-energy molecular orbital had all the phases in the contributing p-orbitals aligned the same way. In other words, there were no nodes between the p-orbitals. But what happens to the molecular orbital diagram if we add a third contributing p-orbital?
AP Chemistry- Practice Bonding Questions for Exam - Quia The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules.
Stereoisomers - Michigan State University Stereoisomers. As defined in an earlier introductory section, isomers are different compounds that have the same molecular formula. When the group of atoms that make up the molecules of different isomers are bonded together in fundamentally different ways, we refer to such compounds as constitutional isomers.For example, in the case of the C 4 H 8 hydrocarbons, …
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Molecular Orbital Theory The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions Experiments have shown that O2 and F2 are best described by the model in the figure above, but B2, C2, and N2 are best described by a model that...
PDF Chapter 5 | 5.2.2 Orbital Mixing The s molecular orbital is a bonding molecular orbital, and has a lower energy than the original atomic orbitals, since this combination of atomic In the p* antibonding case, four lobes result that are similar in appearance to a d orbital, as in Figure 5.2(c). The px, py, and pz orbital pairs need to be...
An introduction to molecular orbital theory Simple molecular orbital diagrams. Dihydrogen and its ion H2+. Dihelium He2. Dilithium Li2. Lithium hydride LiH. Sigma and pi orbitals. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations...
(PDF) Inorganic Chemistry Housecroft | Yurika Almanda ... Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
PDF A qualitFaetIIive molecular orbital diagram SfAoLrC 'sferrocene (D5d) A qualitative molecular orbital diagram for ferrocene (D5d) FeII SALC's Fe. • The attachment of additional groups or ligands destroys the D5d/D5h symmetry of ferrocene thus significantly altering the MO diagram.
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently lower in energy than the valence Does your MO diagram agree with this expectation? Determine the primary MOs that determine the bond order.
By molecular orbital theory, bond order of c2 is 2, while... - Quora This is how the Molecular Orbital diagram for C2 would look like. 2,Molecular Orbital Theory shows that there are two sets of paired electrons in a degenerate pi bonding set of orbitals. This gives a bond order of 2, meaning that there should exist a double bond between the two carbons in a C2...

















0 Response to "38 molecular orbital diagram c2"
Post a Comment