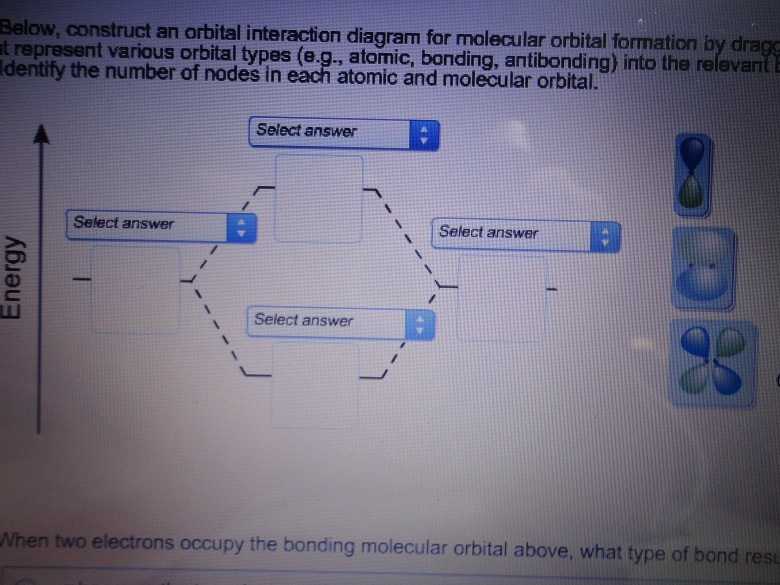
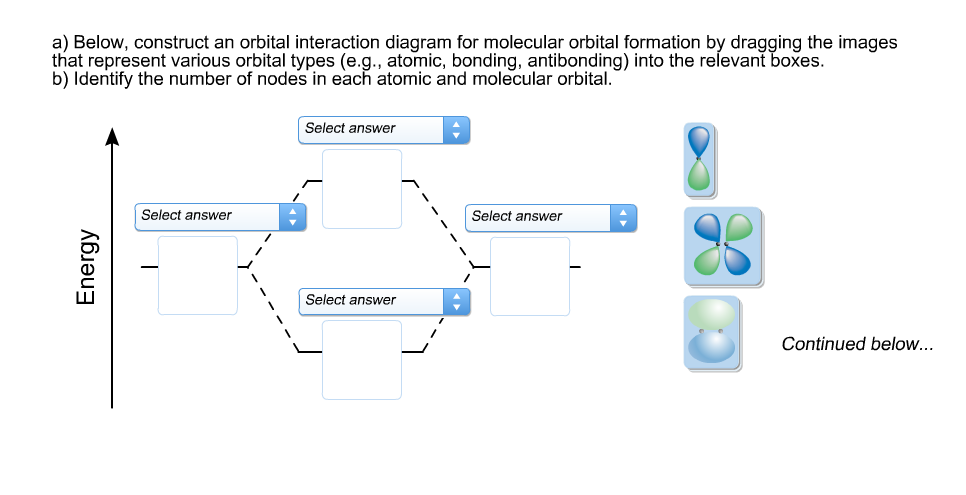
39 below construct an orbital interaction diagram
research.cm.utexas.edu › nbauld › unit1Chapter 1: Molecular Orbital Concepts As an exercise, construct the energy level diagram of butadiene cation radical. Assuming that all of the charge and spin arise from the singly occupied MO (SOMO; a good approximation), calculate the charge(Q i ) and spin (r I ) at each carbon atom (again, you may use symmetry considerations, i.e., C1 = C4 and C2 = C3)). Solved a) Below, construct an orbital interaction diagram ... a) Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbits types (e.g., atomic, bounding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital. c) When two electrons occupy the bounding molecular orbital above, what ...
kledingkeuzes.nl › diagram-of-a-wave-worksheet-answerskledingkeuzes.nl 2 days ago · In this DIAGRAM science station, students diagram different types of waves after doing an activity with rope and a slinky. diagram, and the character maps that we completed to help you study for this test. Body waves inside the earth. In the diagram below, 50% of the light is reflected from the solar panel and 50% is absorbed.
Below construct an orbital interaction diagram
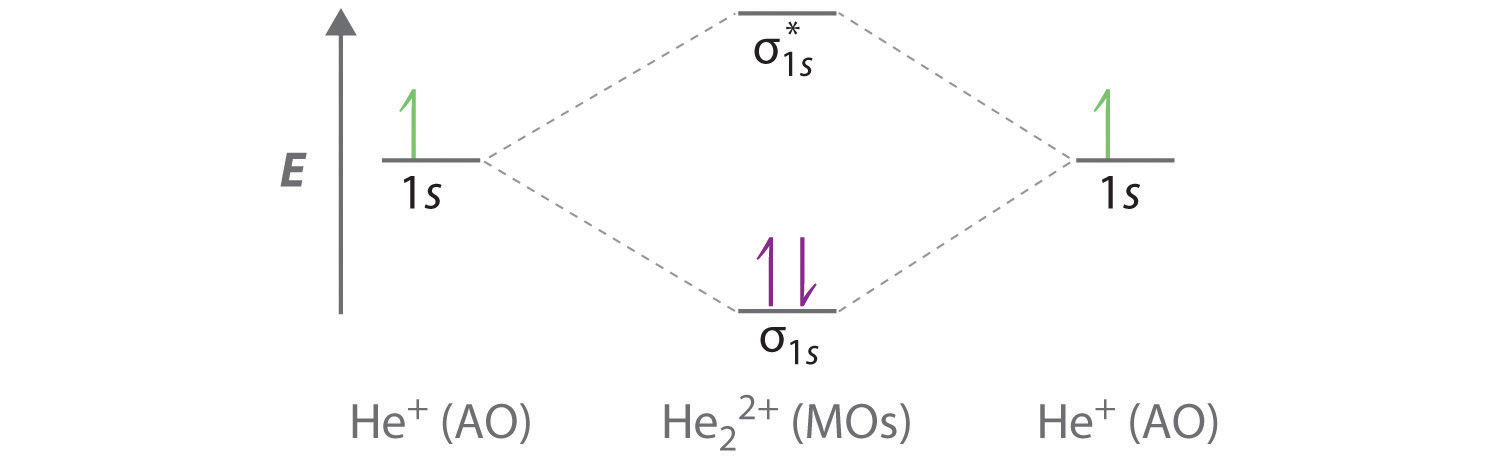
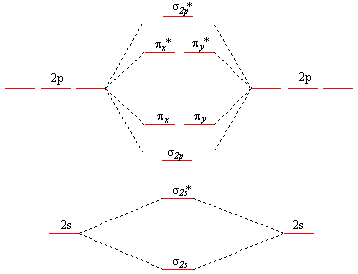
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in ... - CPP The interaction of the two bonded atoms with the bonding electrons produces a more stable arrangement for the atoms than when separated. Electrons usually occupy these orbitals. A sigma bonds is always the first bond formed between two atoms. Sigma star (σ*) antibonding molecular orbital - Normally this orbital is empty, but if it should PDF Another Look at The Covalent Bond: Molecular Orbitals The orbital energies are summarized in an orbital interaction diagram, shown in Fig. 1.14. This diagram is a plot of orbital energy versus the position of the two interact-ing nuclei. The isolated atomic orbitals and their energies are shown on the left and right sides of the diagram, and the molecular orbitals and their energies are shown in ... Solved Below, construct an orbital interaction diagram for ... Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. Identify the number of nodes in each atomic and molecular orbital.
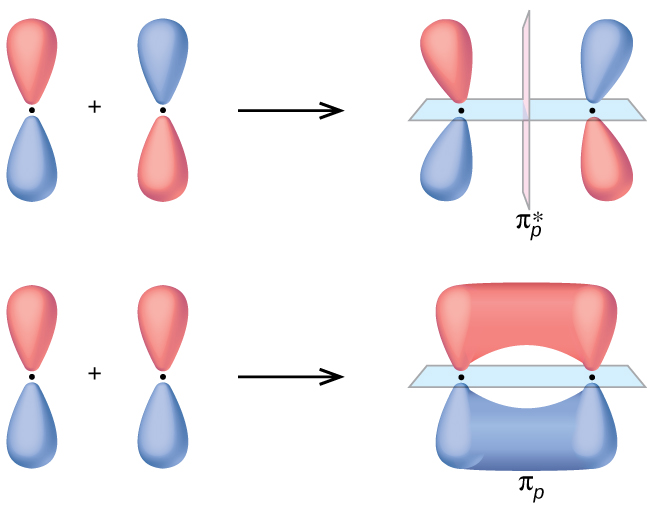
Below construct an orbital interaction diagram. OneClass: Pleaseexplain why for 5 stars. Consider two 2p ... Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital. Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... PDF Orbital Picture of Bonding: Orbital Combinations ... equivalent sp2 orbitals, leaving one p orbital untouched. The process is shown below. 2s 2p X 2p y 2p z Potential energy sp2 hybridization sp2 sp2 sp2 p In this top view, the unhybridized p orbital cannot be seen because it also arranges itself to be as far apart from the sp2 orbitals as possible. PDF Chem 673, Problem Set 5 Due Tuesday, December 2, 2008 (1) tbp (b) Construct an orbital correlation diagram that connects the d orbital levels of an tbp ML5 complex with those for a square pyramidal ML5 complex (again assume the ligands, L, are σ-donors). The diagram depicted above describes the motion that interrelates these two geometries (known as a Berry pseudorotation after Prof. Steven Berry at the ...
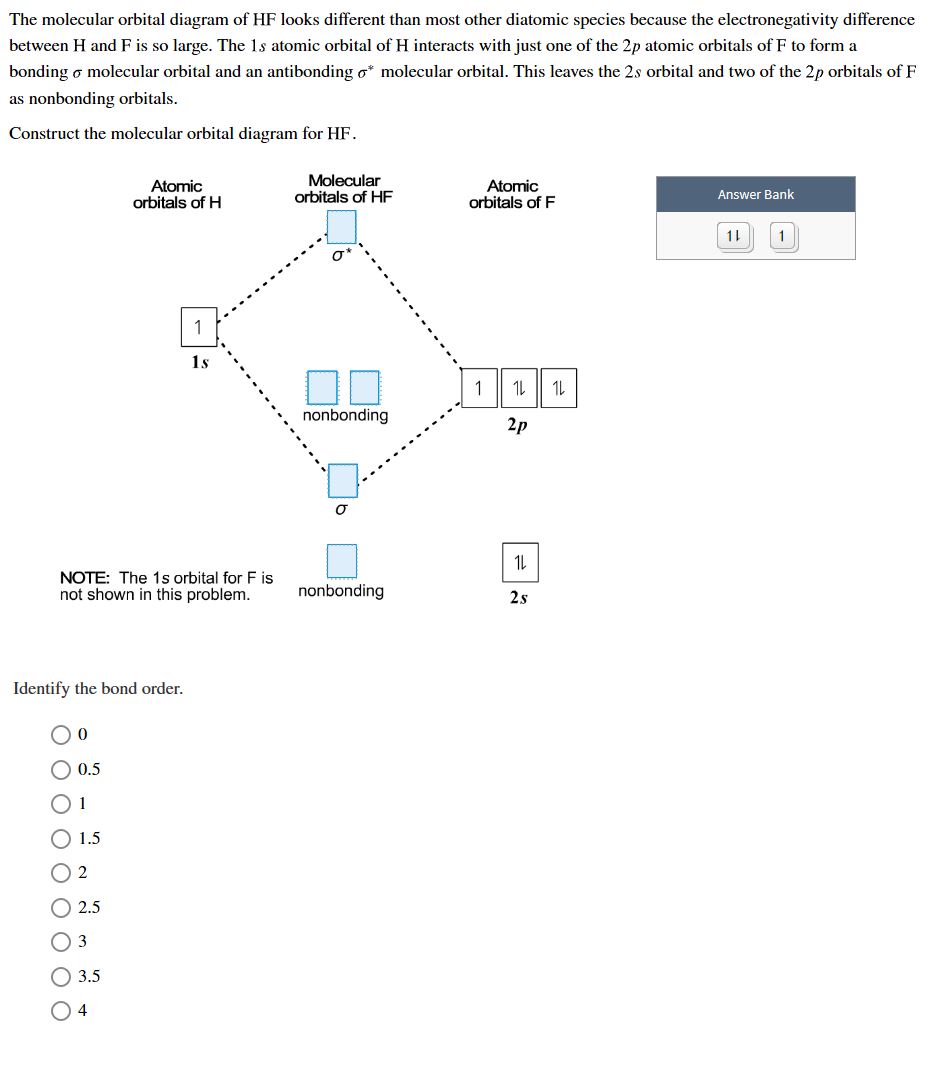
OneClass: molecular orbital diagram Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital. › rgroup › hughbanksReciprocal Space and Brillouin Zones ... - Texas A&M University orbital residing in the unit cell at lattice site R. Since there is only one Bloch basis orbital for this problem, the secular determinant for this problem is trivial, but we still must evaluate H(k): H(k) = e2 ik R R HR; where H = (0)H (R) The diagram here shows the set of coefficients for the factor e2 ik R in this equation (the components of ... PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine OneClass: 12. What is an orbital diagram? Provide an example. An orbital is characterized by a size, shape, and orientation in space below, construct an orbital interaction diagram for molecular Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes.
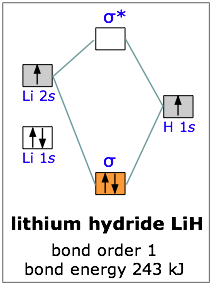
bozeba.de › diagram-of-a-wave-worksheet-answersbozeba.de 2 days ago · Diagram of a wave worksheet answers ... chem.libretexts.org › Bookshelves › General_Chemistry9.8: Molecular Orbital Theory - Chemistry LibreTexts Feb 20, 2022 · The lithium 1s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals. 8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11. OneClass: Below, construct an orbital interaction diagram ... Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital.
PDF ORBITALS and MOLECULAR REPRESENTATION In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
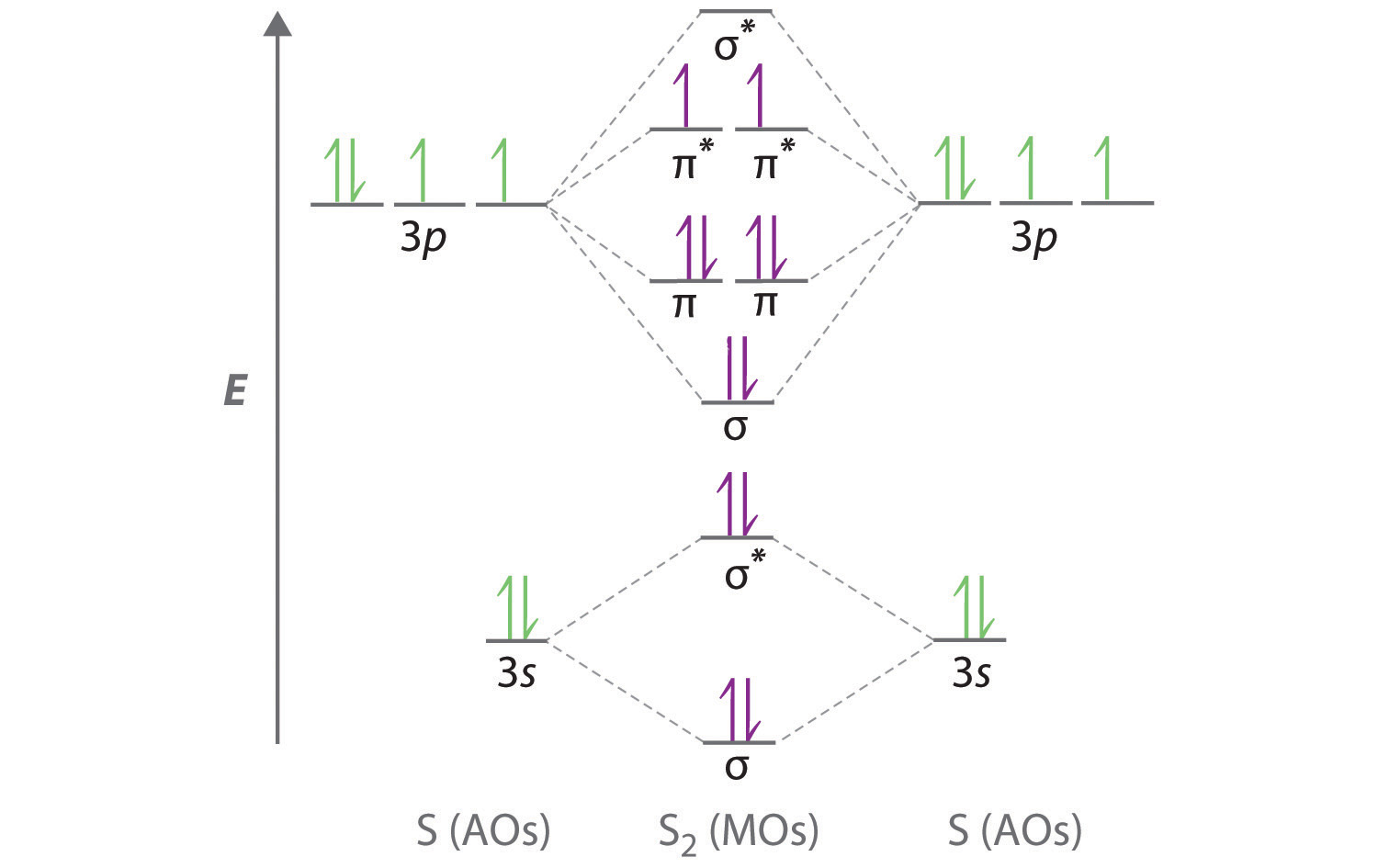
chemed.chem.purdue.edu › genchem › topicreviewMolecular Orbital Theory - Purdue University It is possible for the 2s orbital on one atom to interact with the 2p z orbital on the other. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
Consider two 2p orbitals, one on each of two ... - HomeworkLib Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. Identify the number of nodes in each atomic and molecular orbital. Below, construct an orbital interaction diagram for molecular orbital ...
› 34602222(PDF) Inorganic Chemistry Housecroft | Yurika ... - Academia.edu Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
Pericyclic reactions - Stereoelectronics The interaction diagram below will be used to represent the most ... We can use orbital interaction diagrams to describe the bonding stabilisation that occurs in a transition structure when two different atoms join to form a new bond, and using the same principles it is possible to construct an orbital interaction diagram for two reacting ...
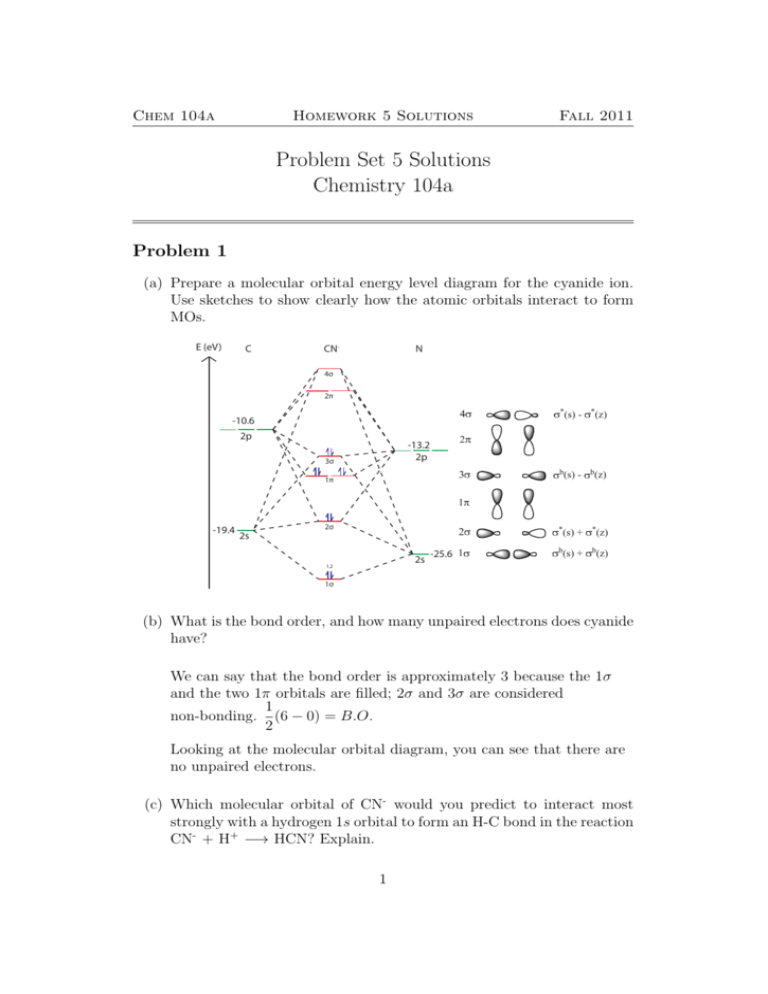
Answered: 4. Construct an orbital interaction… | bartleby Construct an orbital interaction diagram for O2 and predict bond order for: O2, [O2]*, [O2]", and [O2]*. Order the molecules from the shortest to longest O-O bond length. 5. Square planar molecules with formula AB2C2 and octahedral molecules with formulas AB4C2 and AB3C3 feature diastereoisomers. Recall that trigonal bipyramidal geometry ...
Solved a) Below, construct an orbital interaction diagram ... a) Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital.
Molecular Orbitals - Chem1 The lithium 1s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
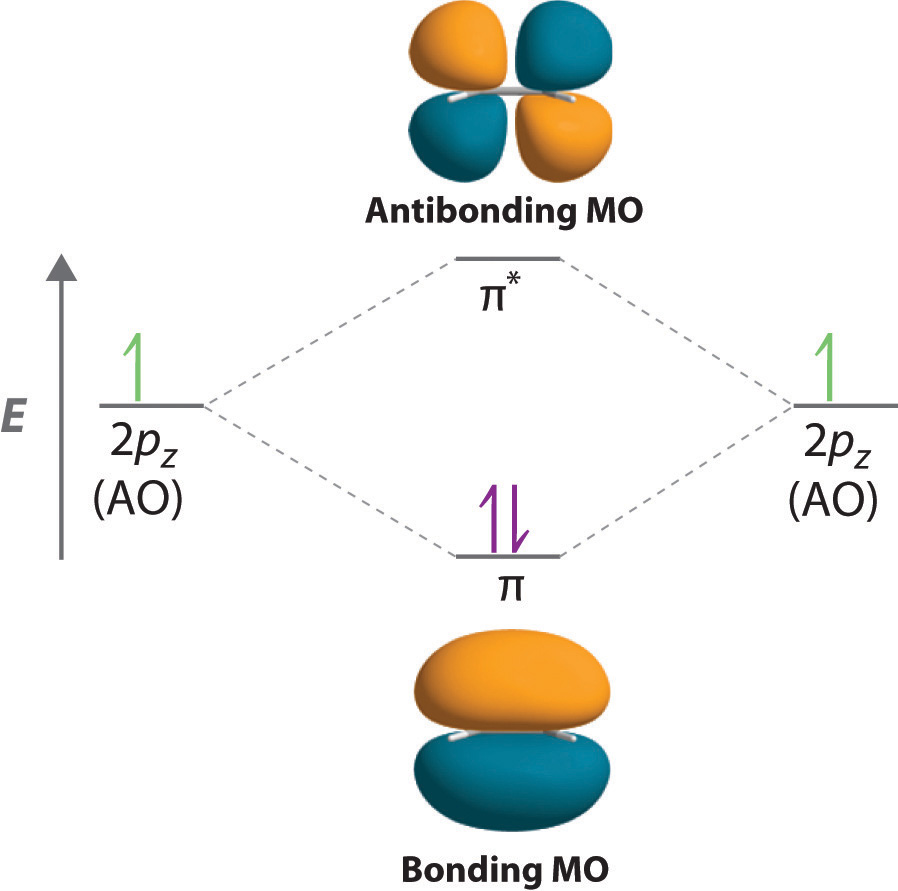
Solved Consider two 2p orbitals, one on each of ... - Chegg Below, construct an orbital interaction diagram for molecular orbital formation Question : Consider two 2p orbitals, one on each of two different atoms, oriented side-to-side, as in the figure. Images bringing these nuclei together so that overlap occurs as shown in the figure.
Solved Below, construct an orbital interaction diagram for ... Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. Identify the number of nodes in each atomic and molecular orbital.
PDF Another Look at The Covalent Bond: Molecular Orbitals The orbital energies are summarized in an orbital interaction diagram, shown in Fig. 1.14. This diagram is a plot of orbital energy versus the position of the two interact-ing nuclei. The isolated atomic orbitals and their energies are shown on the left and right sides of the diagram, and the molecular orbitals and their energies are shown in ...
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in ... - CPP The interaction of the two bonded atoms with the bonding electrons produces a more stable arrangement for the atoms than when separated. Electrons usually occupy these orbitals. A sigma bonds is always the first bond formed between two atoms. Sigma star (σ*) antibonding molecular orbital - Normally this orbital is empty, but if it should
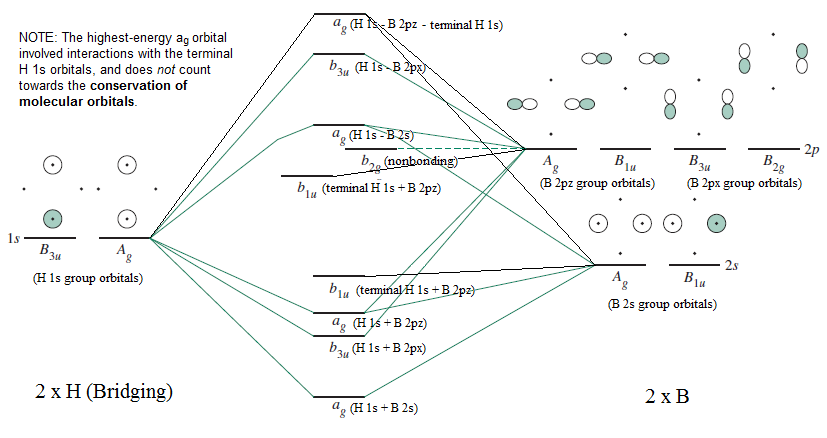






/chapter2/pages17and18/page17and18_files/moconnoncon.png)
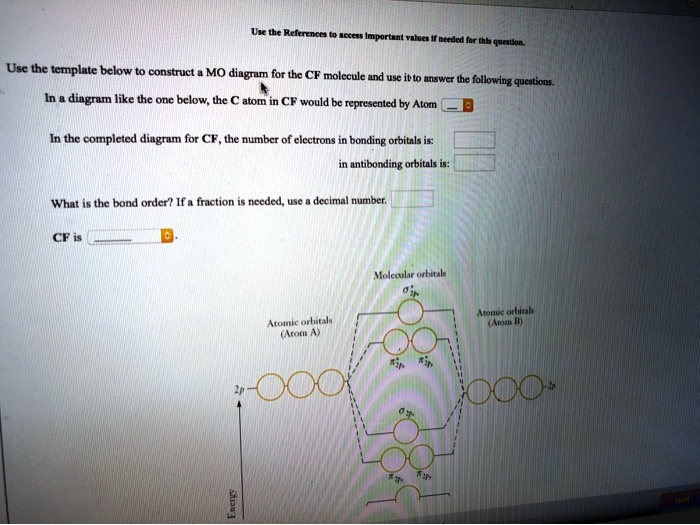













0 Response to "39 below construct an orbital interaction diagram"
Post a Comment