39 in the diagram if electrons flow from the right half
The Student Room The diagram below shows a cell that can be used to measure the standard electrode potential for the half-reaction Fe3+(aq) + e- Fe2+(aq). In this cell, the electrode on the right-hand side is positive. In the diagram attached inside, if electrons flow from the ... Answer Expert Verified 4.6 /5 8 Hagrid In the diagram attached inside, if electrons flow from the right half-cell to the left one through the wire, the statement that is true is that the metal strip on the right has a lower activity than the one on the left. The answer is letter A. Advertisement Answer 5.0 /5 4 Mrknowit Answer: A.
General Chemistry II Jasperse Electrochemistry. Extra ... Which statement is true regarding the direction of electron flow through the external wire? a) Electrons flow from left to right, from the Zinc b) Electrons flow from right to left, to the Zinc c) The zinc electrode will get larger as more zinc forms. d) Anions will flow through the “bridge” from the zinc side to the silver side 22.
In the diagram if electrons flow from the right half
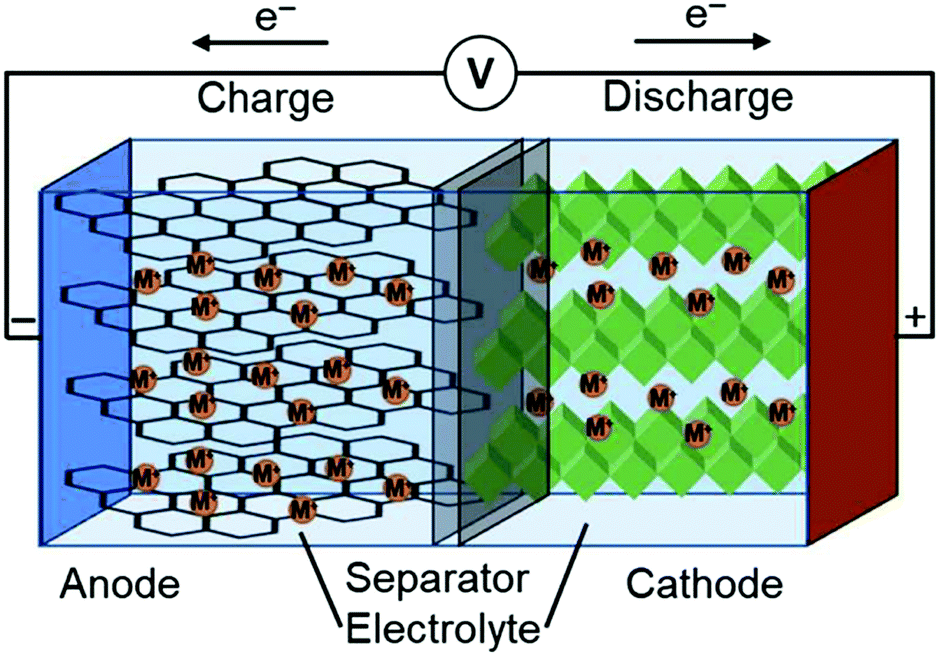
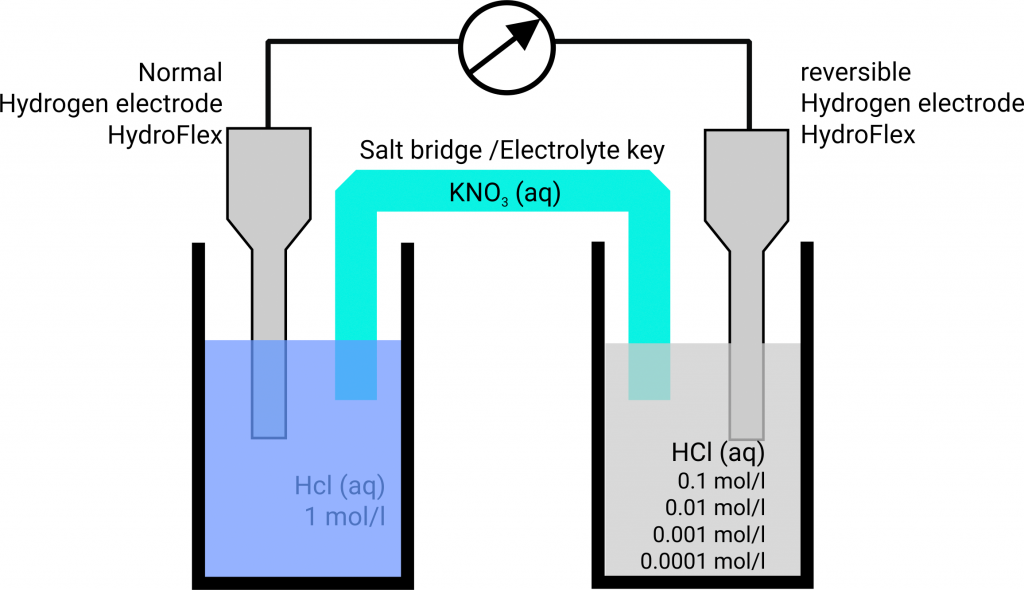
Electrochemical Cell: Definition, Equation, Diagram ... Jan 13, 2022 · Chemical energy drives the flow of electrons in this process, which creates electricity. An ‘Electrochemical cell’ is a device that generates electrical energy from chemical processes within it or uses chemical energy to generate electricity. ... While writing the cell reaction corresponding to a cell diagram, the right-hand side half ... Electrochemistry Basics - Chemistry LibreTexts Aug 07, 2021 · A voltaic cell consists of two compartments called half-cells. The half-cell where oxidation occurs is called the anode. The other half-cell, where reduction occurs, is called the cathode. The electrons in voltaic cells flow from the negative electrode to the positive electrode—from anode to cathode (see figure below). PDF O C R A2 C H E M I S T R Y MODULE 5 - Exam QA Deduce the half-equations for the reactions occurring at the electrodes. ... the electrons flow through the ammeter from right to left. ... €€€€Draw a labelled diagram of a suitable apparatus for the right-hand electrode in this cell. You
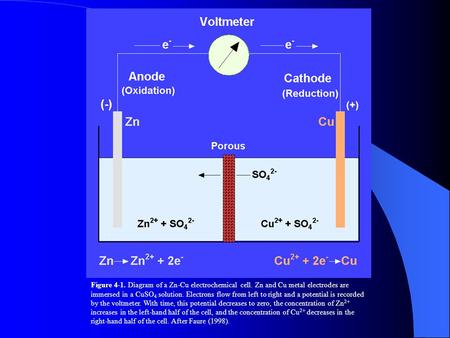
In the diagram if electrons flow from the right half. Bis2A 5.1 REDOX Chemistry and the REDOX Tower - CNX In other words, when electrons flow \"downhill\" in a redox reaction from a compound with a higher (more positive) reduction potential to a second compound with a lower (less positive) reduction potential, they release free energy (review module 4.0 and 4.1). \n \nThe greater the voltage, ΔE 0 ', between the two components, the greater the ... 6.08 unit test p2.pdf - 1 The diagram shows an ... 1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips. In your response, do the following: · Label all parts (1-9), including the solutions in each beaker and the connecting tube. · Label which cell is the cathode and which cell is the anode. Include the charge on each strip. · Show, or describe in detail, the flow of electrons. Solved: Consider the following voltaic cell: (a) In which ... Step 1 of 4. Voltaic cell: A voltaic cell is also known as galvanic cell. It performs the function of converting chemical energy into electrical energy. It consists of two electrodes that are present at the left hand side known as anode and at the right hand side known as cathode. Chapter 21, Problem 30P is solved. View this answer. Electrochemistry: Galvanic Cells and the Nernst Equation The electrons flow from the oxidation reaction (Zn(s)--> Zn 2+ (aq) + 2 e-) to the reduction half-cell (Cu 2+ (aq) + 2 e---> Cu(s)) So the electrons flow from the left to the right. Can you identify the flow of ions in the salt bridge? Because negative electrons are flowing through the wire to the reduction half-cell, positive ions travel ...
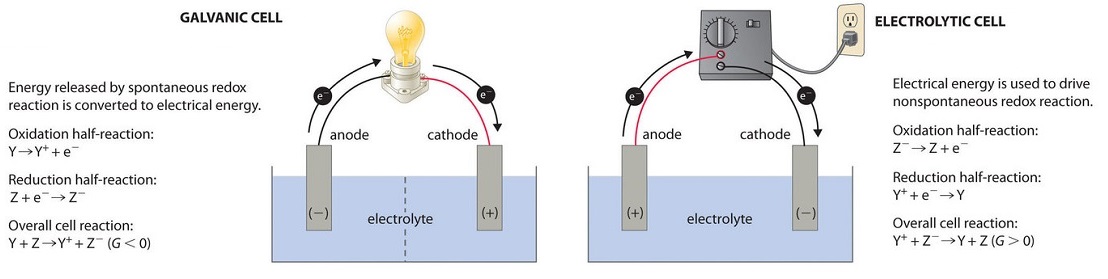
6.08 Unit Test_ Electrochemistry - Part 2 (1).docx - 1 ... The diagram shows an electrochemical cell with a gold strip (left) and aluminum glasses (right). In your response, do the following: • Label all parts. • Label the cathode and the anode, including the charge on each. • Show the flow of electrons. • Describe what type of electrochemical cell is pictured and what its use is. • Explain ... Voltaic Cell Basics | Other Quiz - Quizizz Question 3. SURVEY. 30 seconds. Q. In a voltaic cell, the half cell that receives anions from the salt bridge is. answer choices. cathode. anode. Tags: Welcome to CK-12 Foundation | CK-12 Foundation FlexBook® Platform, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation. Voltaic Cells - University of Texas at Austin Below is an animated diagram of a voltaic cell showing electrons leaving the anode (on the left for oxidation), going through the wire, maybe a voltmeter, and then entering the cathode (on the right for reduction). Remember that in a voltaic cell, the spontaneous chemical reaction (redox rxn) is "pushing" and "pulling" those electrons through ...
Which Way Does Electricity Flow? - Douglas Krantz Electron Flow Most technicians think that electricity flows in a wire the direction that electrons flow. This is the direction in a wire that actually shows physical movement. A major advantage to this thinking is that in a wire, magnetism is affected by the movement of electrons. (Magnetism is a major effect of electromagnetism). Electrochemical Cells | Boundless Chemistry Reduction describes the gain of electrons by a molecule, atom, or ion. The electrons always flow from the anode to the cathode. The half-cells are connected by a salt bridge that allows the ions in the solution to move from one half-cell to the other, so that the reaction can continue. Key Terms Galvanic Cell | Dornshuld whereas the cathode is represented by the right-half of the cell diagram and demonstrates the reduction half-reaction Ag + ( a q) + e − Ag ( s) The double vertical bars in the middle of the cell diagram represents the salt bridge. Electrons flow from left-to-right (anode-to-cathode) in the cell diagram. Concentration PDF 1. Compared to the number of free electrons in a conductor ... the current can flow (4) they have a large number of free electrons 3. If an insulator replaces a conductor in an electrical circuit, the flow of electrons in the circuit will be (1) less (3) the same (2) greater 4. When the total resistance of a simple electrical circuit is decreased while keeping the voltage
Electrochemistry: cells and electrodes "Conventional current flow" is from positive to negative, which is opposite to the direction of the electron flow. This means that if the electrons are flowing from the left electrode to the right, a galvanometer placed in the external circuit would indicate a current flow from right to left.
Electrochemical Cells - University of Texas at Austin The righthand side is the reduction half where the copper 2+ ions are being reduced to copper metal. The solid piece of copper is the cathode. The two sides are connected by a wire that allows electrons to flow from the anode to the cathode. They are also connected by a salt bridge.
How do you draw a cell diagram in chemistry? Why do electrons flow from anode to cathode? By definition, a cathode is a negatively charged electrode (a metal plate or a wire), and an anode is a positively charged electrode. Therefore, electrons are repelled by the cathode and are attracted to the anode, which results in the current of electrons flowing from the cathode to the anode.
AC generation - Energy Education Figure 2. A simplified diagram of an AC generator. The right half of the armature is moving left, while the left half is moving right. Therefore, the electromotive force on the right side is directed towards the near end of the armature, and the force on the left side of the armature is directed towards the far end of the armature. This allows a current to flow through the loop, as …
17.2 Galvanic Cells – Chemistry - opentextbc.ca Electrons flow from the anode to the cathode: left to right in the standard galvanic cell in the figure. The electrode in the left half-cell is the anode because oxidation occurs here. The name refers to the flow of anions in the salt bridge toward it. The electrode in the right half-cell is the cathode because reduction occurs here.
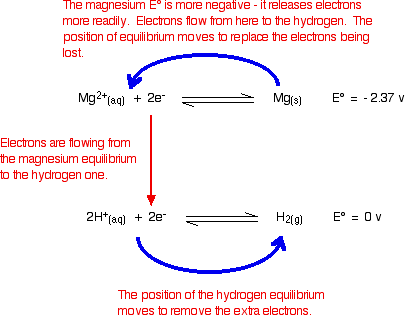
redox (electrode) potentials and test tube reactions Electrons will continue to flow and the two equilibria will again turn into one-way reactions to give the electron-half-equations we've just used to build the ionic equation. Showing that again: Note: People sometimes worry whether the fact that one of the equations has to be multiplied by two affects the argument in any way.
In the diagram attached inside, if electrons flow from the ... SHOW ANSWER In the diagram attached inside, if electrons flow from the right half-cell to the left one through the wire, the statement that is true is that the metal strip on the right has a lower activity than the one on the left. The answer is letter A. Answer from: Quest SHOW ANSWER It changes food into energy that the organism can use.
The diagram shows an electrochemical cell with copper ... Answers: 3 on a question: The diagram shows an electrochemical cell with copper (left) and zinc (right) strips. In your response, do the following: Label all parts (1-9), including the solutions in each beaker and the connecting tube. Label which cell is the cathode and which cell is the anode. Include the charge on each strip. Show, or describe in detail, the flow of electrons. Describe ...
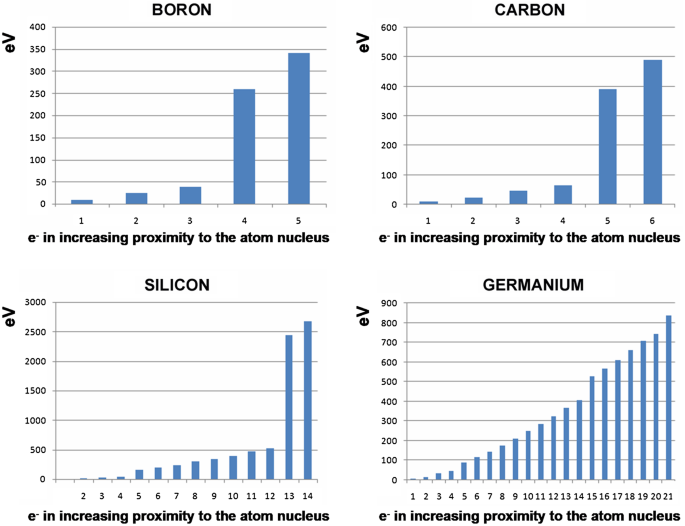
P-type Semiconductor : Doping, Energy Diagram & Its Conduction The PN-junction diode is made up of two adjacent parts of two semiconductor materials like p-type and n-type. These materials are semiconductors like Si (silicon) or Ge (germanium), including atomic impurities. Here the type of semiconductor can be determined by the kind of impurity there. The procedure of adding impurities to semiconductor materials is known as …
what is a cell diagram - shapovmusic.com To write the cell reaction corresponding to a cell diagram, the right-hand half reaction is written as a reduction, and the left-hand half-reaction, written as an oxidation, is added to it. (This is exactly the same as subtracting the left-hand equation written as a reduction, which is the formally correct procedure.) ... The electrons flow ...
Chemistry 3 - Redox 1 Flashcards - Quizlet A current of electrons will flow from the more reactive metal to the less reactive one (electrochemical cell). There is an equilibrium position closer to the less reactive metal. A potential difference exists between each half cell.
How Voltaic Cells Function - Department of Chemistry Click the diagram to see working cell; click the mouse to reset. Notice that aqueous potassium sulfate {2 K +, SO 4-2} was used in the salt bridge.Potassium ions {K +} diffuse from the salt bridge to replace copper(II) ions {Cu 2+} as the ions are reduced (2 potassium ions for every copper(II) ion).In the other half cell, sulfate ions {SO 4 2-} diffuse from the salt bridge to balance the ...
Chemical reactions for the energy transition | MIT Energy ... Focusing just on the electrons, if the reaction-to-product conversion at the left sends the same number of electrons per second into the "bath of electrons" in the catalyst composite as the oxygen-to-water conversion at the right takes out, the two half-reactions will be balanced, and the electron flow—and the rate of the combined ...
Solved Reduction Half-Reaction EV Ag + AR 0.80 Al + 3 ... Label the diagram above with the chemicals requested below so that electrons flow through the wire in the direction shown (from the electrode on the left to the electrode on the right.) The colors shown here are to help distinguish one cell from the other; Question: Reduction Half-Reaction EV Ag + AR 0.80 Al + 3€ → Ali -1.66 3. A working ...
PDF The Bronx High School of Science On the given diagram, indicate with one or more arrows the direction ofelectron flow through the wire. Explain the function of the salt bridge in the given diagram. Write an equation for the half-reaction that occurs at the zinc electrode in the given diagram. Name: 1109 - 1 -Page 1 electrical energy, nonspontaneously nuclear energy ...
In the diagram attached inside, if electrons flow from the ... In the diagram attached inside, if electrons flow from the right half-cell to the left one through the wire, which of the following statements is true? A. The metal strip on the right has a lower activity than the one on the left. B. The activities of both metal strips are equal. C.
The Cell Potential - Chemistry ... - Chemistry LibreTexts May 05, 2021 · The cell potential, \(E_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Electrons are able to move between electrodes because the chemical reaction is a redox reaction.
CHM112 chapter 21 Flashcards - Quizlet 1.00 moles of Zn and 0.500 moles of Zn2+ ions in 1.00 L of solution are used to form one half-reaction cell, while 2.00 moles of Cu and 3.00 moles of Cu+ in 1.00 L of solution are used to form a second half-reaction cell. The equation for the overall cell reaction is given by Zn (s) + 2Cu+ (aq) → Zn2+ (aq) + 2Cu (s).
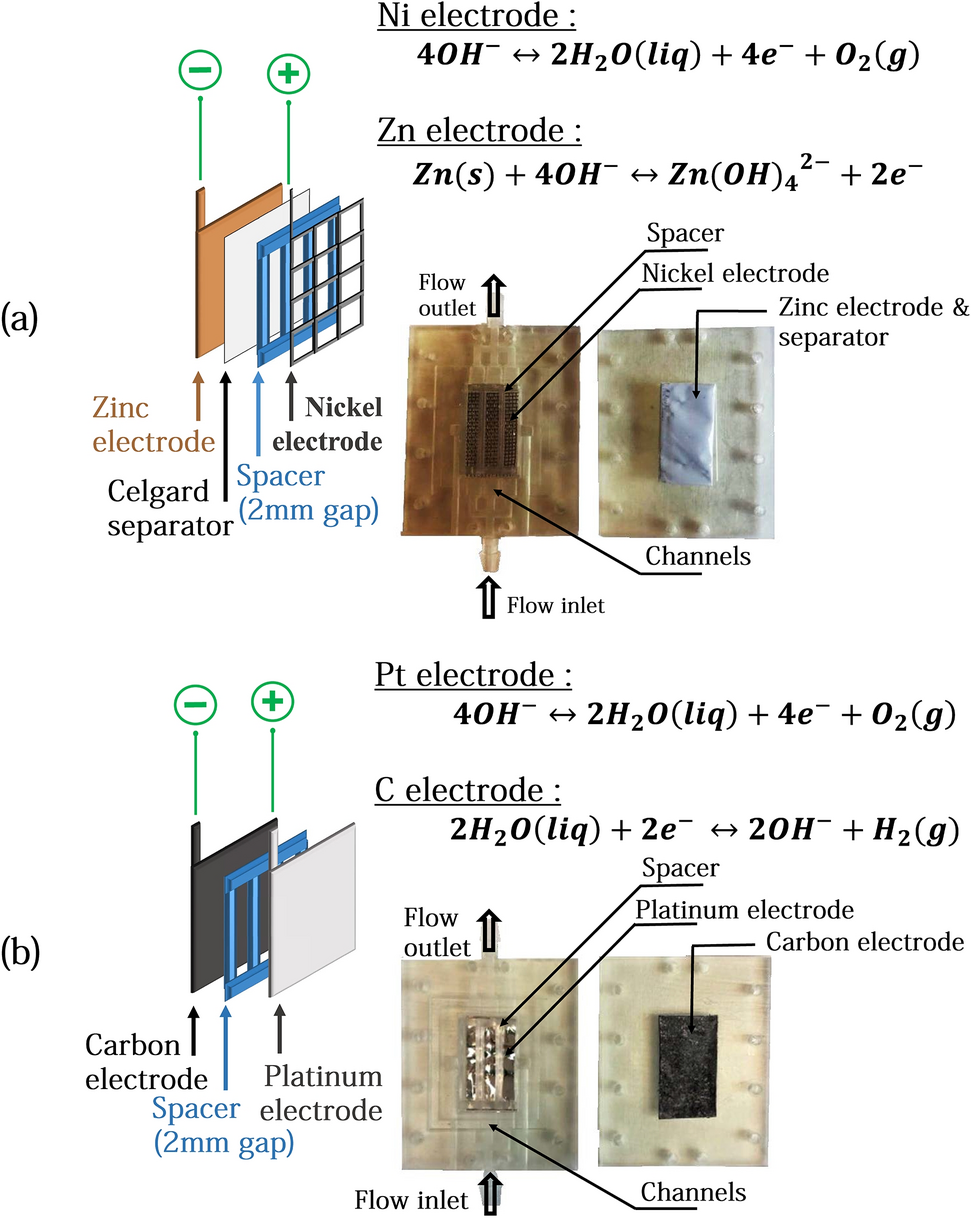
A Brief on Paper Battery Construction and Working - ElProCus Electronic devices and gadgets require a power supply (either AC or DC), this power supply can be taken directly from the mains power supply or from the electrical batteries. The battery can be defined as an electronic device comprised of (one or more) electrochemical cells. The chemical energy of the electrochemical cells can be converted into electrical energy.
PDF First rule: Arrows are used to indicate movement of electrons depict the flow or movement of electrons during chemical reactions. Arrow pushing helps chemists keep track of the way in which electrons and their associated atoms redistribute as bonds are made and broken. The first essential rule to keep in mind is the following: First rule: Arrows are used to indicate movement of electrons
PDF O C R A2 C H E M I S T R Y MODULE 5 - Exam QA Deduce the half-equations for the reactions occurring at the electrodes. ... the electrons flow through the ammeter from right to left. ... €€€€Draw a labelled diagram of a suitable apparatus for the right-hand electrode in this cell. You
Electrochemistry Basics - Chemistry LibreTexts Aug 07, 2021 · A voltaic cell consists of two compartments called half-cells. The half-cell where oxidation occurs is called the anode. The other half-cell, where reduction occurs, is called the cathode. The electrons in voltaic cells flow from the negative electrode to the positive electrode—from anode to cathode (see figure below).
Electrochemical Cell: Definition, Equation, Diagram ... Jan 13, 2022 · Chemical energy drives the flow of electrons in this process, which creates electricity. An ‘Electrochemical cell’ is a device that generates electrical energy from chemical processes within it or uses chemical energy to generate electricity. ... While writing the cell reaction corresponding to a cell diagram, the right-hand side half ...


















0 Response to "39 in the diagram if electrons flow from the right half"
Post a Comment