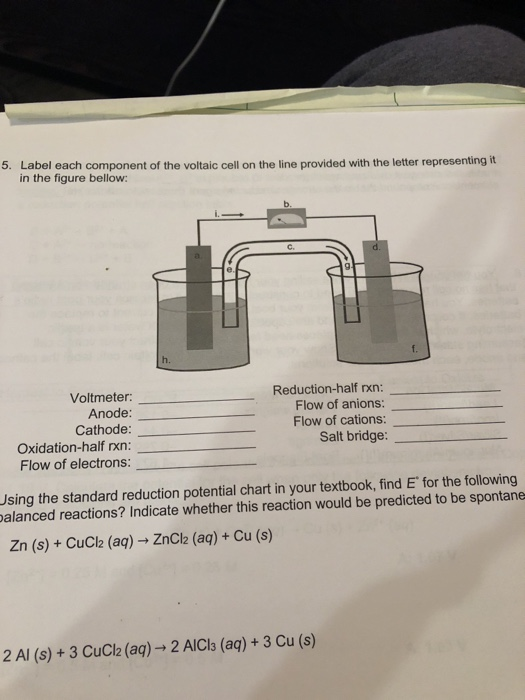
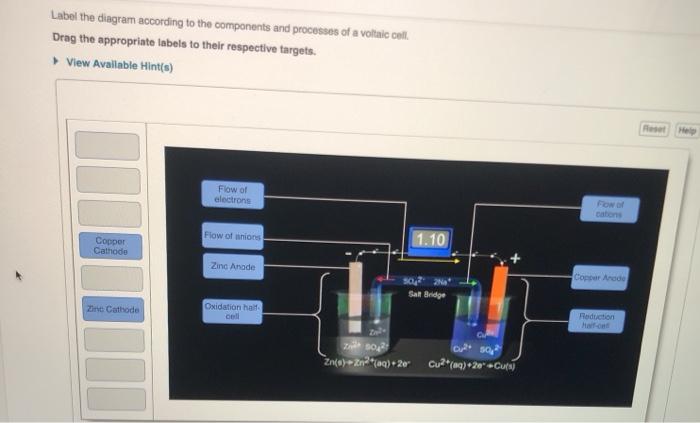
39 label the diagram according to the components and processes of a voltaic cell
Solution: In this electrochemical cell, wh... | Clutch Prep Q. Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. Q. Identify the location of oxidation in anelectrochemical cell. A) the salt bridge B) the socket C) the electrode D) the anode E) the cathode ... Galvanic Cells (Voltaic Cell) - Definition, Working ... Principle of Galvanic (Voltaic) Cell. Electric work done by a galvanic cell is mainly due to the Gibbs energy of spontaneous redox reaction in the voltaic cell. It generally consists of two half cells and a salt bridge. Each half cell further consists of a metallic electrode dipped into an electrolyte. These two half-cells are connected to a ...
Label the diagram according to the compone... | Clutch Prep Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate label to their respective targets. Learn this topic by watching Galvanic Cell Concept Videos
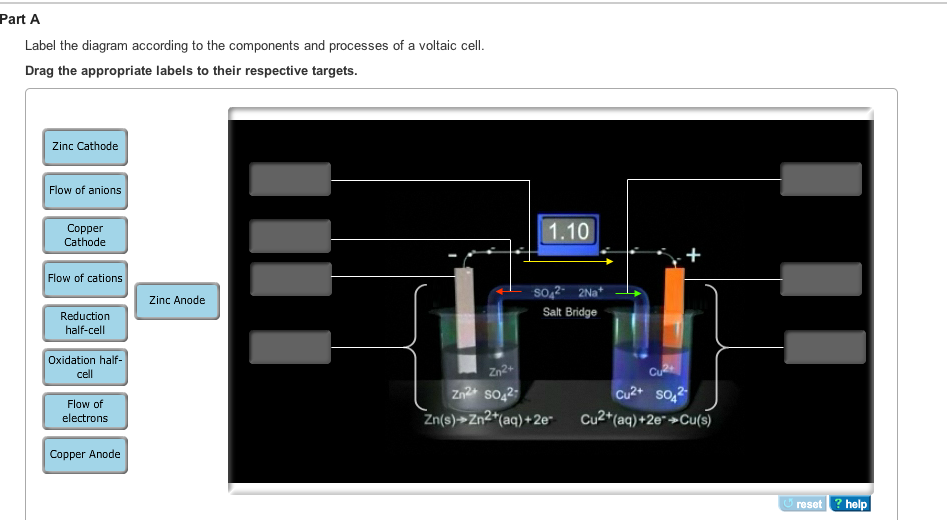
Label the diagram according to the components and processes of a voltaic cell
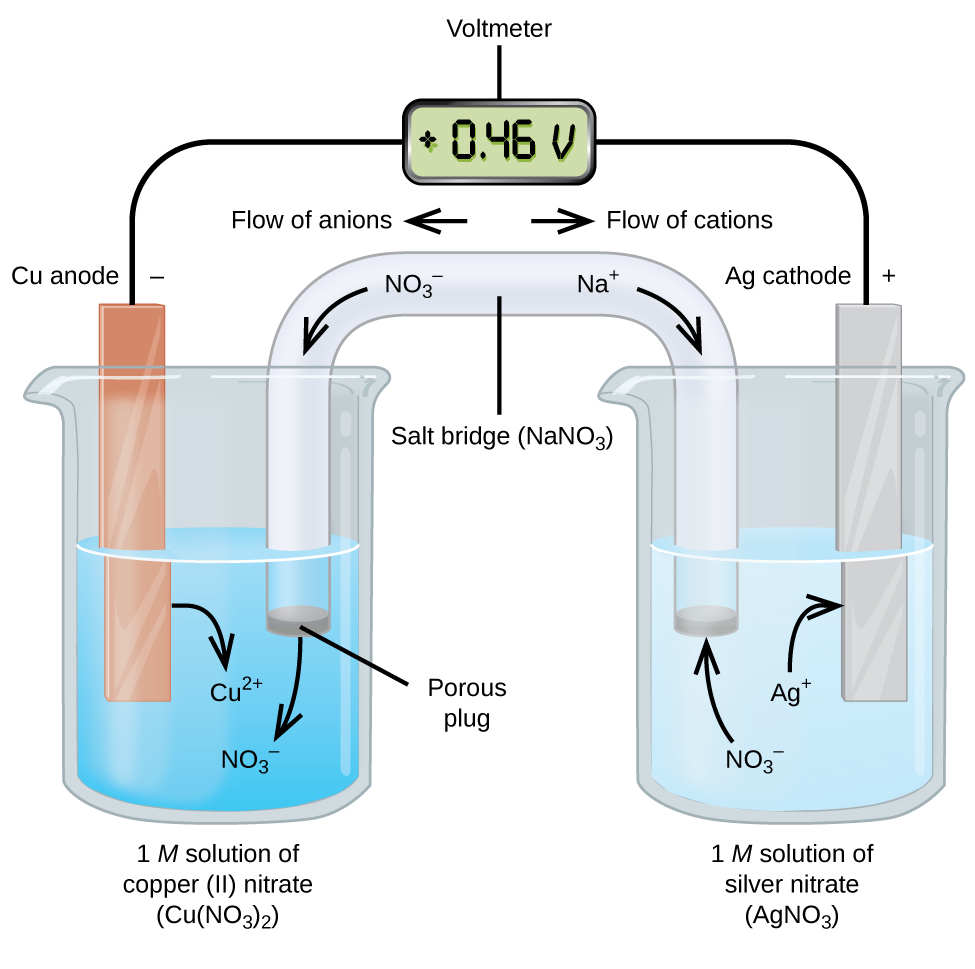
OneClass: The diagram that follows represents a molecular ... If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A. Label the diagram according to the components and processes of a voltaic cell. OneClass: A voltaic cell is constructed based on the ... If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A. Label the diagram according to the components and processes of a voltaic cell. A Well Labelled Diagram And Parts Of An Autoclave Results DIAGRAM OF AUTOCLAVE WITH LABEL on qp ... This article defines the principle of sterilization by autoclave. Label the diagram according to the components and processes of a voltaic cell. Drag the ... Get A Quote
Label the diagram according to the components and processes of a voltaic cell. Solution: What is the name given to the ex ... - Clutch Prep Q. Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. Q. In this electrochemical cell, what is the anode? 1. The solid zinc electrode 2. The Zn2+(aq) ions in the 1 M solution 3. The Cu2+(aq) ions in the... Label The Diagram According To The Components And ... Label the diagram according to the components and processes of a voltaic cell. The other is a copper metal strip in a solution of a copper salt. The electrode at which reduction occurs electrons flow into the cathode electrodes. The voltaic cell is a method to separate these two reactions and create these currents from moving electrons. Watch the video that describes the cell reaction and the ... If a copper-zinc voltaic cell utilizes and solution, you will use a saturated solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective ... Galvanic Cell Video & Text Solutions For College Students ... Q. Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate label to their respective targets. Solved • Mar 4, 2020
OneClass: For electrochemical analysis, samples should be ... If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A. Label the diagram according to the components and processes of a voltaic cell. How do you sketch galvanic cells? + Example - Socratic.org Sketch a cell diagram for the reaction. Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s) Solution: A. Draw one of the diagrams above (no labels). B. Identify what is oxidized and reduced. Zn is oxidized; Cu²⁺ is reduced. C. Put the oxidation materials in the left hand cell. Label the Zn as the negative anode. In the solution put Zn²⁺ and NO₃⁻. Which metal dissolves in HNO3 but not in H ... - Clutch Prep Q. Label the diagram according to the components and processes of voltaic cell. Drag the appropriate labels to their respective targets. Drag the appropriate labels to their respective targets. Q. Cathodic protection of iron involves using another more reactive metal as a sacrificial anode. A weight lifter bench‑presses a 160.0 kg 160.0 kg barbell ... A weight lifter bench‑presses a 160.0 kg 160.0 kg barbell from his chest to a height equal the length of his arms, 0.800 m . 0.800 m. How much work does he do? work: J J What is the potential energy of the barbell relative to the weight lifter's chest after he lifts the barbell? potential energy: J J How many food calories does the weight lifter use if he does this lift 35 times?
Solved Label the diagram according to the components and ... Best Answer. This is the best answer based on feedback and ratings. 100% (103 ratings) Transcribed image text: Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. Previous question Next question. OneClass: Which statement concerning the cathode in an ... If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A. Label the diagram according to the components and processes of a voltaic cell. Drawing & Labeling a Diagram of a Voltaic Cell | Study.com Let's quickly review what a redox (reduction and oxidation) reaction is. This is when one compound gives up electrons to another compound. So, the compound that gives up electrons is oxidized while the compound that accepts electrons is reduced, making it a reduction and oxidation reaction. Typically, when this occurs in solution the energy transfer is simply lost in heat. But if we could capture that energy then we could have an alternative energy source. One way that we create energy is by creating currents, or moving electrons. When a redox reaction occurs we can think about it as though two reactions are occurring, two half-reactions. In the first reaction the compound being oxidized gives up 2 electrons, making extra electrons on the products side. And in the second reaction the compound being reduced accepts 2 electrons, requiring extra electrons on the reactant side. These types of reactions are called half-reactions. Animation-Analysis of Copper-Zinc Voltaic ... | Clutch Prep If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4 solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments or two half-cells.Part A. Label the diagram according to the components and processes of a voltaic cell.
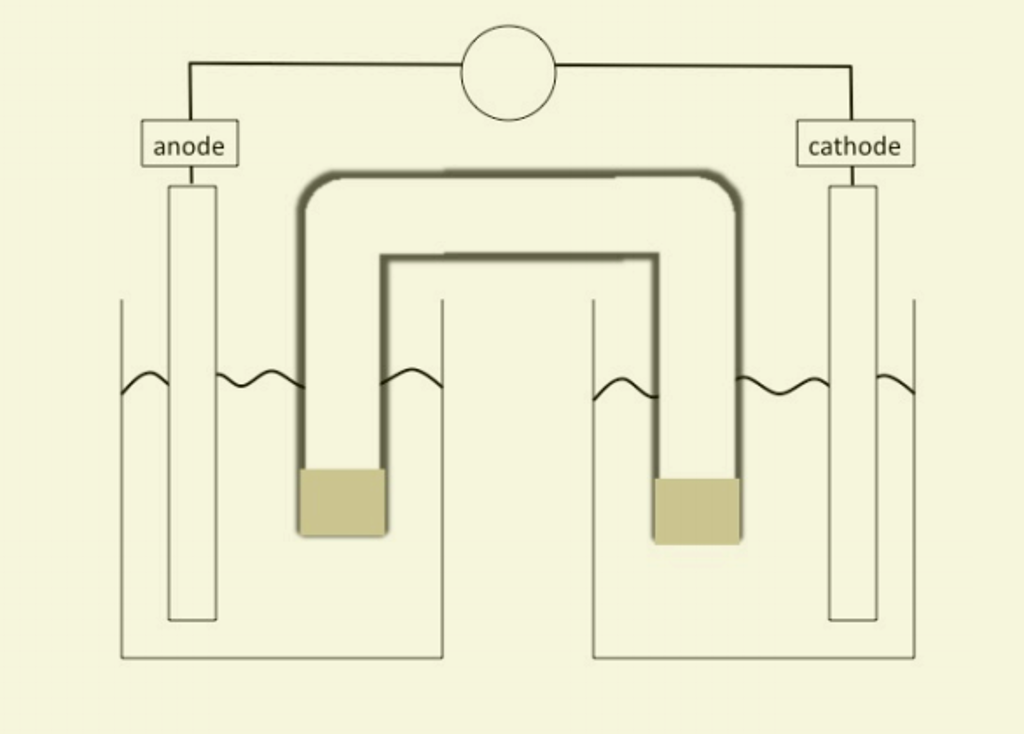
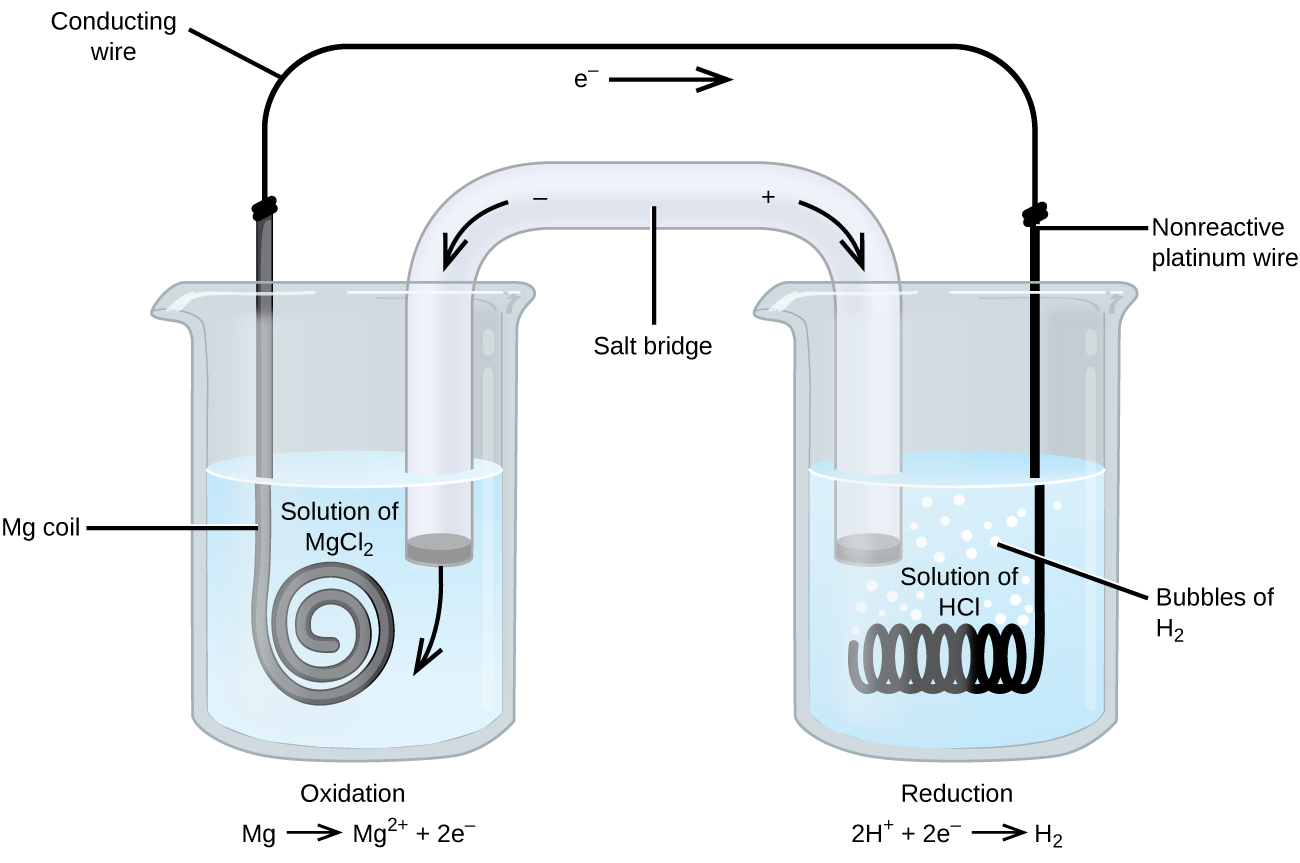
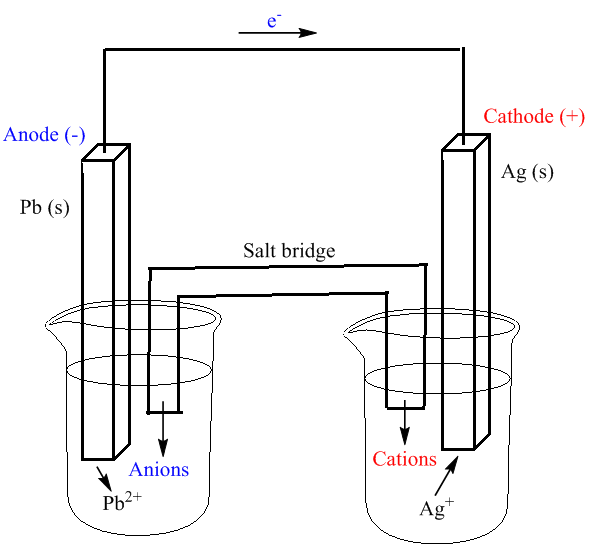
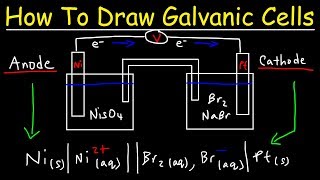
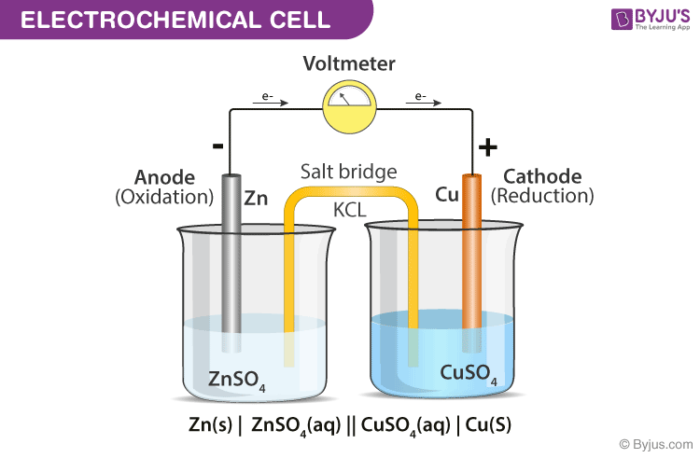
Label each half cell in this diagram of a voltaic cell to ... Apr 21, 2020 · The cell as shown is a voltaic cell. A voltaic cell produces electrical energy by a spontaneous chemical reaction. Oxidation occurs at the anode and reduction occur at the cathode. The electrode at the left hand side the anode where oxidation occurs while the electrode at the right hand side is the cathode where reduction occurs.
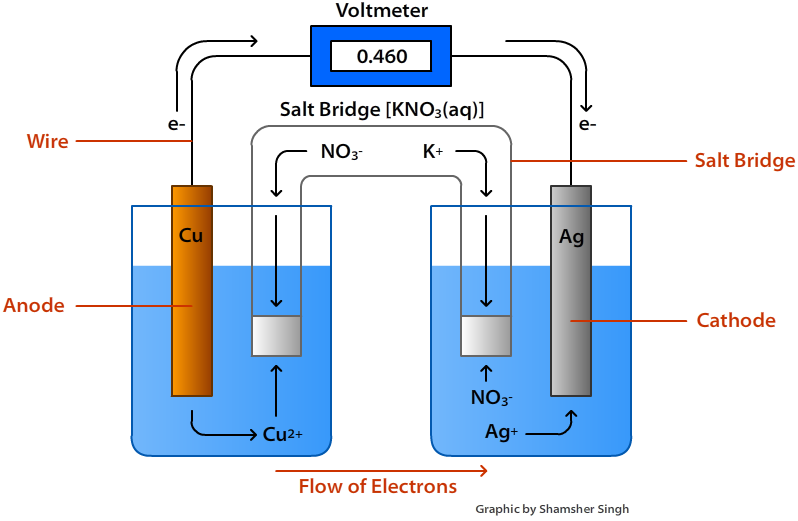
Chem 180 Exam 3 Flashcards - Quizlet Label the diagram according to the components and processes of a voltaic cell. The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the cobalt-silver voltaic cell.
Solved Label the diagram according to the components and ... Solved Label the diagram according to the components and | Chegg.com. Science. Chemistry. Chemistry questions and answers. Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. Question: Label the diagram according to the components and processes of a voltaic cell.
OneClass: You are asked to build an electrolytic cell ... If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A. Label the diagram according to the components and processes of a voltaic cell.
Voltaic Cells - Department of Chemistry Goal: to describe the construction and operation of a voltaic cell Working Definitions. Electrical current is the movement of charged particles, either electrons or ions, through a conductor.. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The important parts of a voltaic cell:. The anode is an electrode where oxidation occurs.
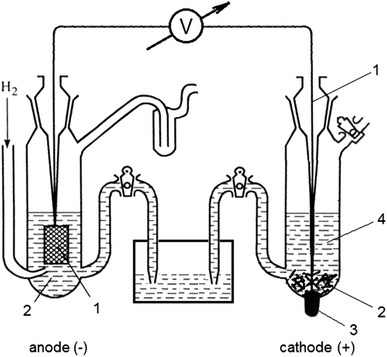
Study 96 Terms | Chemistry Flashcards - Quizlet the half-cell electrode that is normally chosen to have a potential of zero (kind of an arbitrary zero to measure the other electrode potentials against because we can only measure the overall potential that occurs when two half-cells are combined in a whole cell); consists of an inert platinum electrode immersed in 1 M HCl with hydrogen gas at 1 atm bubbling through the solution
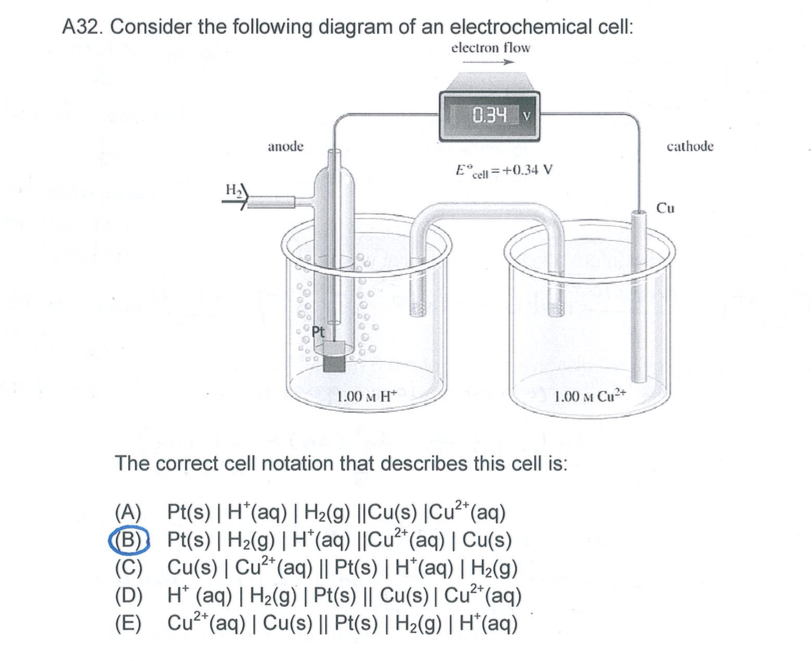
Answered: A voltaic electrochemical cell is… | bartleby Transcribed Image Text: A voltaic electrochemical cell is constructed using the following reaction. The half-cell components are separated by a salt bridge. 2cl'(aq) + F2(g)- Cl2(g) + 2F'(aq) Write the reactions that take place at the anode and at the cathode, the direction in which the electrons migrate in the external circuit, and the direction the anions in the salt bridge migrate.
Chapter 17: Electrochemistry Flashcards | Quizlet Chapter 17: Electrochemistry. Label the diagram according to the components and processes of a voltaic cell. The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the ...
SOLVED:Sketch a voltaic cell for each redox reaction ... this question deals with electro chemistry and with voltaic cells, those are spontaneous electrochemical cells. And the first example were asked to look at is a reaction between lead and silver. And what we're told according to what forms in the process here is that led undergoes this half reaction gives up two electrons to form the lead, two plus cat eye on and those electrons get transferred ...
A Well Labelled Diagram And Parts Of An Autoclave Results DIAGRAM OF AUTOCLAVE WITH LABEL on qp ... This article defines the principle of sterilization by autoclave. Label the diagram according to the components and processes of a voltaic cell. Drag the ... Get A Quote
OneClass: A voltaic cell is constructed based on the ... If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A. Label the diagram according to the components and processes of a voltaic cell.
OneClass: The diagram that follows represents a molecular ... If a copper-zinc voltaic cell utilizes ZnSO4 and CuSO4solution, you will use a saturated Na2SO4 solution in the salt bridge. Thus, the salt bridge will help the migration of ions across the two compartments, or two half-cells. Part A. Label the diagram according to the components and processes of a voltaic cell.


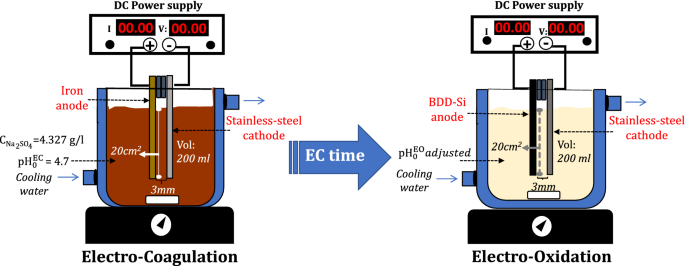
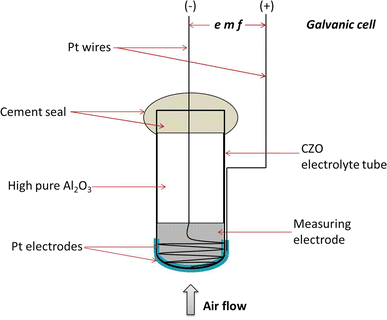
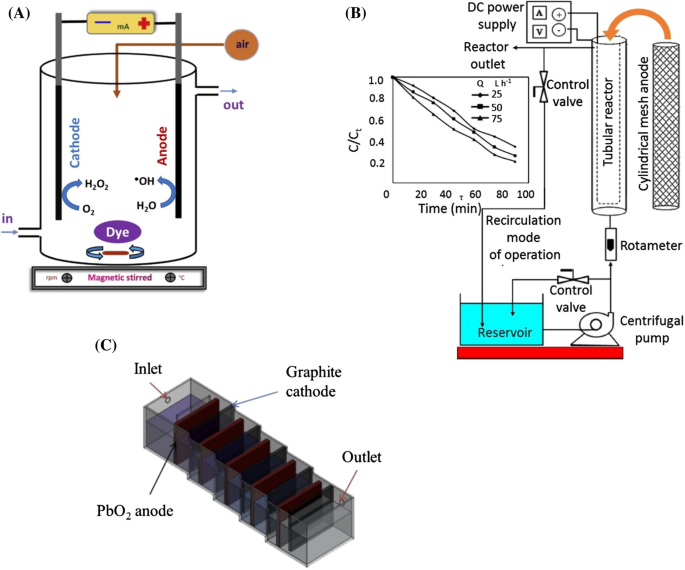
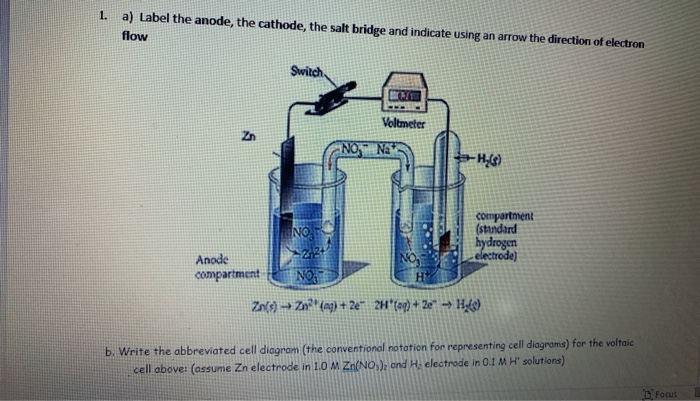











0 Response to "39 label the diagram according to the components and processes of a voltaic cell"
Post a Comment