39 lewis diagram for so2
What is the Lewis dot structure of so2? - AskingLot.com SO2 Lewis structure. To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each. Click to see full answer. SiO2 Lewis Structure, Molecular Geometry, Hybridization ... Silicon Dioxide (SiO2) Lewis Structure. The Lewis structure of SiO2 is identical to the Lewis structure of CO2. The only difference is that instead of carbon, silicon is used. One silicon atom is at the middle, with two oxygen atoms bound to it in a double bond. There are no lone pairs on the central atom of the SiO2 Lewis dot structure,
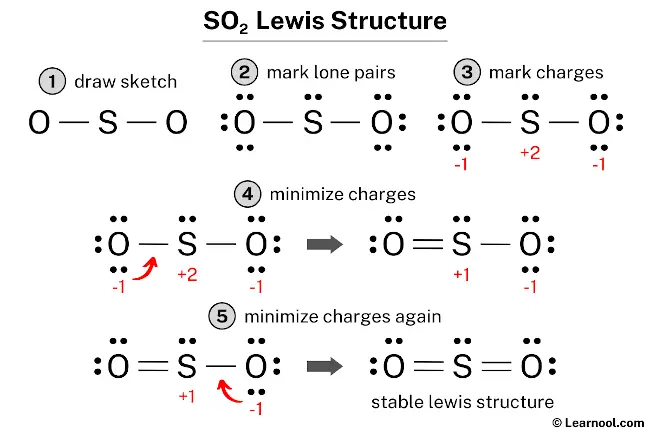
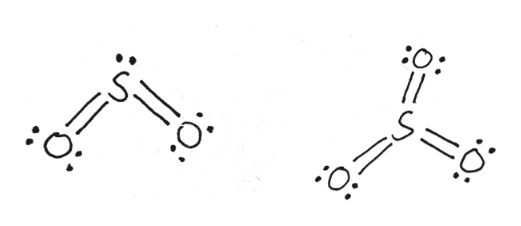
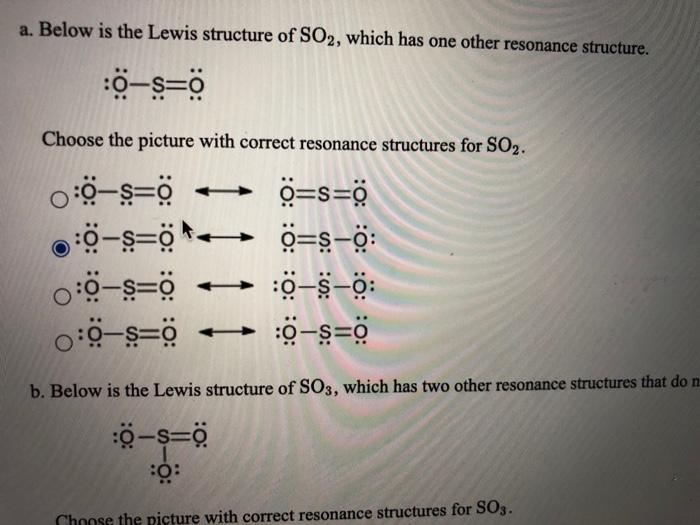
Lewis Diagram For So2 - schematron.org Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms.
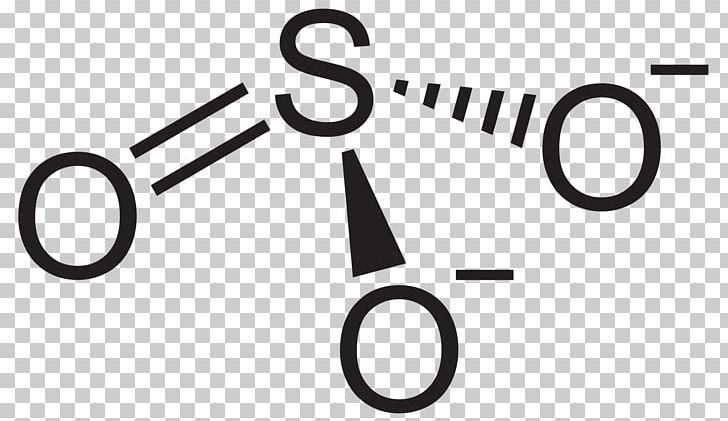
Lewis diagram for so2
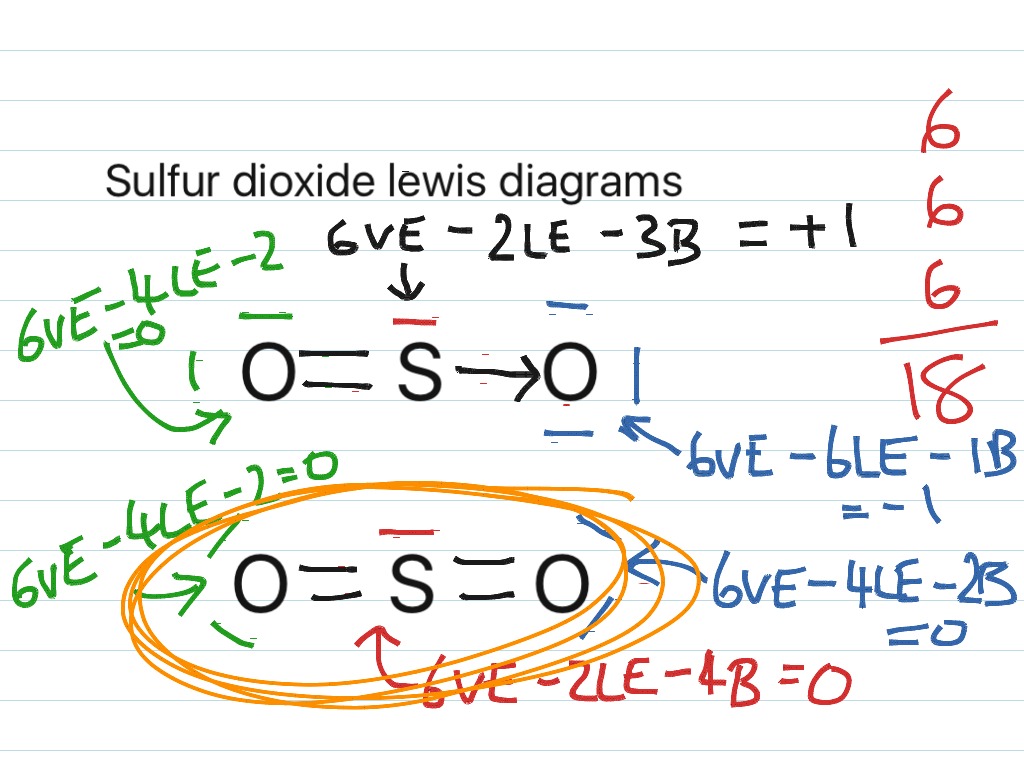
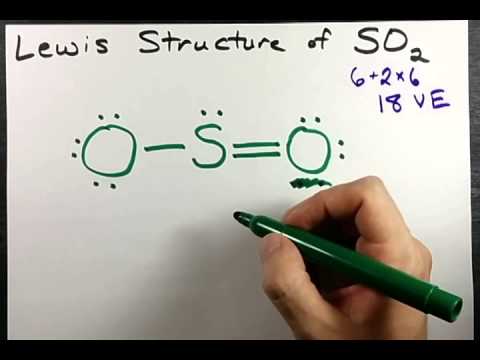
SO2 Lewis Structure, Hybridization, Molecular Geometry ... Now let's see the lewis structure of SO2. In SO2, the sulfur's valence electron = 6 And the valence electrons of oxygen = 6 There are 2 oxygen atoms in the compound, thus = 6*2 = 12 So, total valence electrons = 18 After drawing the skeletal structure, we can see that none of the atoms can fulfill their octet with single bonds. SO2(Sulfur Dioxide) Lewis Structure, Hybridization ... This is a theoretical structure obtained using formal charges- this is the structure that we will take to be Sulfur Dioxide's final Lewis structure. However, it is worth noting that in an experimental sense (data and tools), we find single and double bonds present in the SO 2 structure. The final Lewis structure for SO 2 is shown below. It ... Lewis structure calculator | Lewis structure generator To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO4- 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button.
Lewis diagram for so2. SO2 Molecular Geometry, Hybridization, Lewis Structure ... Now let's see the lewis structure of SO2. In SO2, the sulfur's valence electron = 6 And the valence electrons of oxygen = 6 There are 2 oxygen atoms in the compound, thus = 6*2 = 12 So, total valence electrons = 18 After drawing the skeletal structure, we can see that none of the atoms can achieve their octet with single bonds. So2 Lewis Dot Diagram - schematron.org To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each. 70 More Lewis Dot Structures. S does not follow the octet rule. How to draw SO2 Lewis Structure? - Science Education and ... In the SO2 Lewis structure diagram, we always begin by introducing valence electrons from the central sulfur atom (in step1). As a result, wrap around the central sulfur atom's bond pair valence electrons first (see figure for step1). The sulfur atom in the molecule gets only 10 electrons around its molecular structure. Lewis structure of SO2 - CHEMISTRY COMMUNITY Re: Lewis structure of SO2. Postby Sanskriti Balaji 1J » Sat Dec 04, 2021 10:36 am. Hi! Either structure works for SO2 since it has resonance! To figure out the dominant structure, you would have to find the formal charge of each structure using this formula: (Valence Electrons) - (Lone Pairs + Bonded Pairs/2)!
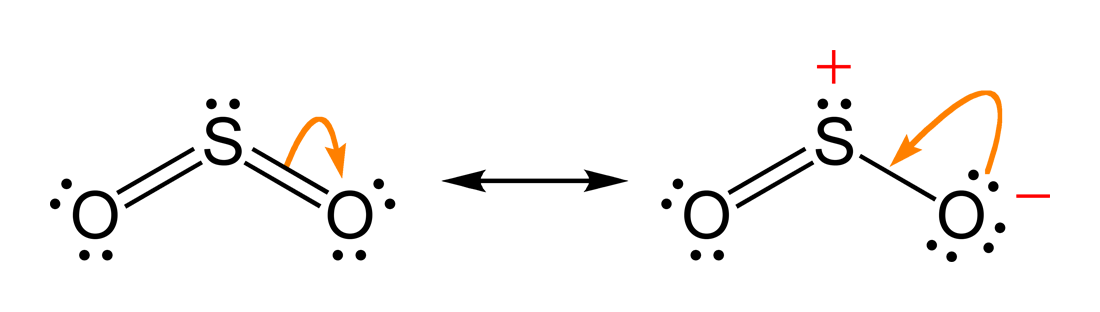
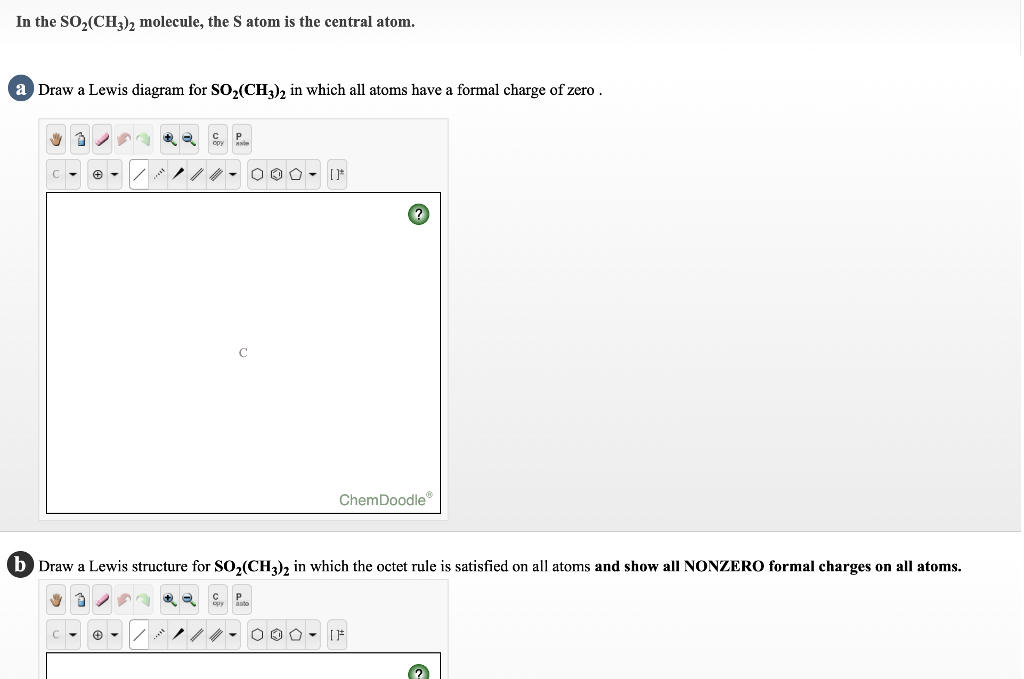
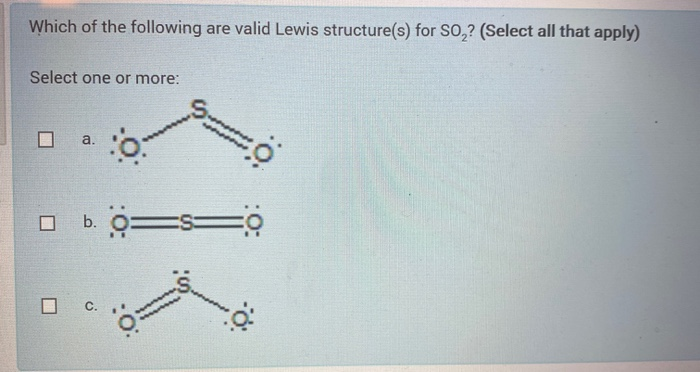
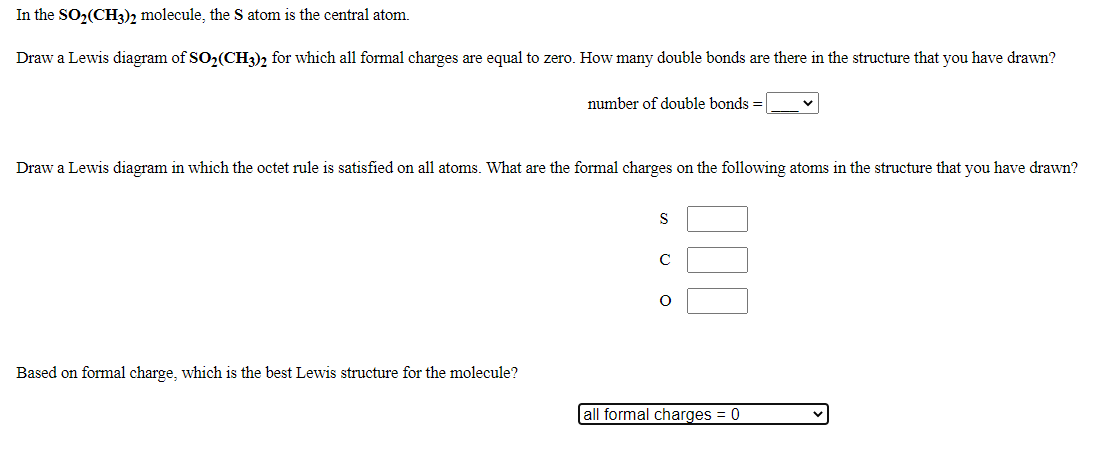
NO2 Lewis Structure: Complete Guide (2022 Updated) NO2 Lewis Structure: Complete Guide (2022 Updated) Nitrogen Dioxide (NO2) is one of the covalent compounds containing a central nitrogen atom bonded to an oxygen atom and double-bonded to another oxygen atom. Our team spent a few hours researching to give you information about the NO2 Lewis structure. Lewis Structure of SO2 - CHEMISTRY COMMUNITY Re: Lewis Structure of SO2. From what I understand, all of those lewis structures are valid. They're essentially resonance structures, but the first structure you mentioned, with two double bonds, is the preferred one. This is because all formal charges are zero. In the structure with one single bond and one double bond, the Sulfur has a +1 ... Solved In the SO2(CH3)2 molecule, the Satom is the central ... In the SO2(CH3)2 molecule, the Satom is the central atom. Draw a Lewis diagram of SO2(CH3)2 for which all formal charges are equal to zero. How many double bonds are there in the structure that you have drawn? number of double bonds = Draw a Lewis diagram in which the octet rule is satisfied on all atoms. PDF LEWIS DIAGRAMS - Colorado State University Lewis diagrams, you would fi nd that the triangular form for ozone works well but it is excluded by experimental evidence. Therefore we must procede to work out the linear alternative: There are a total of 6 x 3 = 18 valence electrons, of which 4 are used in the single bonds, leaving 14. By
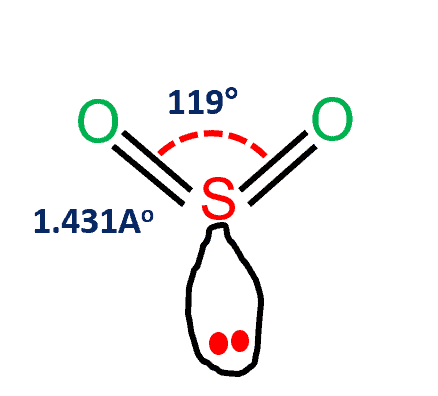
SO2 Lewis Structure - How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth... Lewis Structure of SO2 (sulfur dioxide) - YouTube How to draw the Lewis Structure of SO2 - with explanationCheck me out: Draw Lewis electron dot structure of SO2 - Chemistry ... Draw Lewis electron dot structure of SO2 . Maharashtra State Board HSC Science (Electronics) 11th. Textbook Solutions 6926. Important Solutions 18. Question Bank Solutions 4571. Concept Notes & Videos 336. Syllabus. Advertisement Remove all ads. Draw Lewis electron dot structure of SO2 - Chemistry ... SO2 Lewis Structure - Lewis Dot Structure | Chem Helps Speaking for the SO2 Lewis Structure, one of the oxygen atoms is attached to the sulfur atom with a single bond and the other with a double bond. Single bonds should be longer than double bonds. However, in the measurements made, it was determined that both bonds had the same length (143 pm).
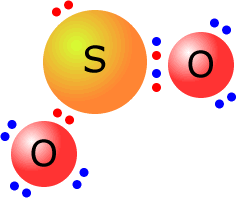
SO2 Lewis Structure - Learnool SO2 Lewis Structure SO 2 (sulfur dioxide) has one sulfur atom and two oxygen atoms. In the lewis structure of SO 2, there are two double bonds around the sulfur atom, with two oxygen atoms attached to it. On each oxygen atom, there are two lone pairs, and on the sulfur atom, there is one lone pair. Steps
Lewis Structure of SO2 (2021 UPDATED) Complete Guide With SO2 Lewis Structure, the central atom is the central sulfur atom because of the higher valence of the sulfur atom than the oxygen atom. The SO2 Lewis Structure provides the best explanation of how the sulfuric acid (1) transformed into such after dissecting the bonds of Sulfur and Oxygen. This can be a hazard to one's health, but this is ...
Lewis diagrams (practice) | Khan Academy Lewis diagrams. Drawing Lewis diagrams. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Exceptions to the octet rule. Worked example: Lewis diagram of xenon difluoride (XeF₂) Practice: Lewis diagrams. This is the currently selected item. Next lesson.
CO2 Lewis Structure - Lewis Dot Structure | Chem Helps CO2 Lewis Dot Structure When drawing the Lewis structure of the carbon dioxide molecule, the carbon and an unpaired electron of oxygen share with each other. As a result, a single covalent bond between carbon and oxygen occurs. However, in this case, carbon and oxygen cannot complete the octet. For carbon and oxygen to complete…
Dot Diagram For So2 - Wiring Diagrams Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. It is known as a formula written as SO2.
SO2 Lewis Structure, Molecular Geometry, Hybridization ... Steps for drawing Lewis dot structure of SO2 Step 1: Count the total number of valence electrons In the first step, we have to find out how many valence electrons are present in the given molecule. In the case of SO2, there is one sulfur and two oxygen atoms are present.
What is the correct Lewis structure for so2? SO2 Lewis structure. To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. You know that both the Sulphur and Oxygen has six valence electrons each. Here we have two Oxygen atoms, so a total number of valence electrons will be eighteen.
Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan ... The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
Sulfur dioxide (SO2) Lewis Structure, Hybridization Lewis structure of SO 2 There are two double bonds between sulfur atom and oxygen atoms in SO molecule. Also, a lone pair exists on sulfur atom and each oxygen atom has two lone pairs in SO 2 lewis structure. Hybridization of SO 2 All atoms have sp 2 hybridization. Each oxygen atom has one sigma bond and two lone pairs.
Draw a lewis structure for so2 in which all atoms obey the ... Draw a Lewis structure for SO2 in which all atoms have a formal charge of zero. Explicitly showing the zero charges is optional. Do not consider ringed structures. Answer. General guidance. Concepts and reason. Calculate total number of valance electrons in . and draw the Lewis structure that contains all the atoms with octet configuration.
Lewis Structure of SO2 - Chemistry Stack Exchange Lewis Structure of SO2 [duplicate] Ask Question Asked 4 years, 3 months ago. Modified 4 years, 3 months ago. Viewed 53k times 3 $\begingroup$ This question already has answers here: Hybridization of sulfur in sulfur dioxide (2 answers) Closed 4 years ago. What is the structure of ...
Lewis structure calculator | Lewis structure generator To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO4- 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button.
SO2(Sulfur Dioxide) Lewis Structure, Hybridization ... This is a theoretical structure obtained using formal charges- this is the structure that we will take to be Sulfur Dioxide's final Lewis structure. However, it is worth noting that in an experimental sense (data and tools), we find single and double bonds present in the SO 2 structure. The final Lewis structure for SO 2 is shown below. It ...
SO2 Lewis Structure, Hybridization, Molecular Geometry ... Now let's see the lewis structure of SO2. In SO2, the sulfur's valence electron = 6 And the valence electrons of oxygen = 6 There are 2 oxygen atoms in the compound, thus = 6*2 = 12 So, total valence electrons = 18 After drawing the skeletal structure, we can see that none of the atoms can fulfill their octet with single bonds.




















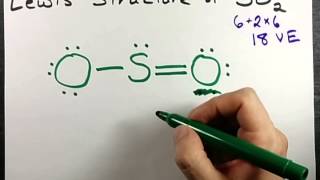
0 Response to "39 lewis diagram for so2"
Post a Comment