39 orbital diagram for scandium sc
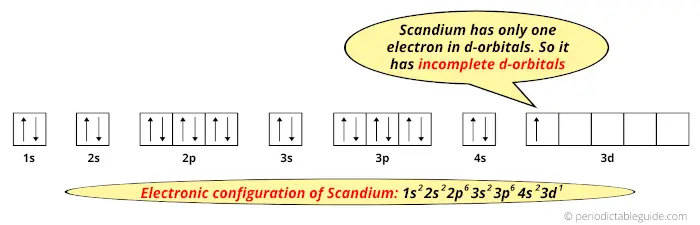
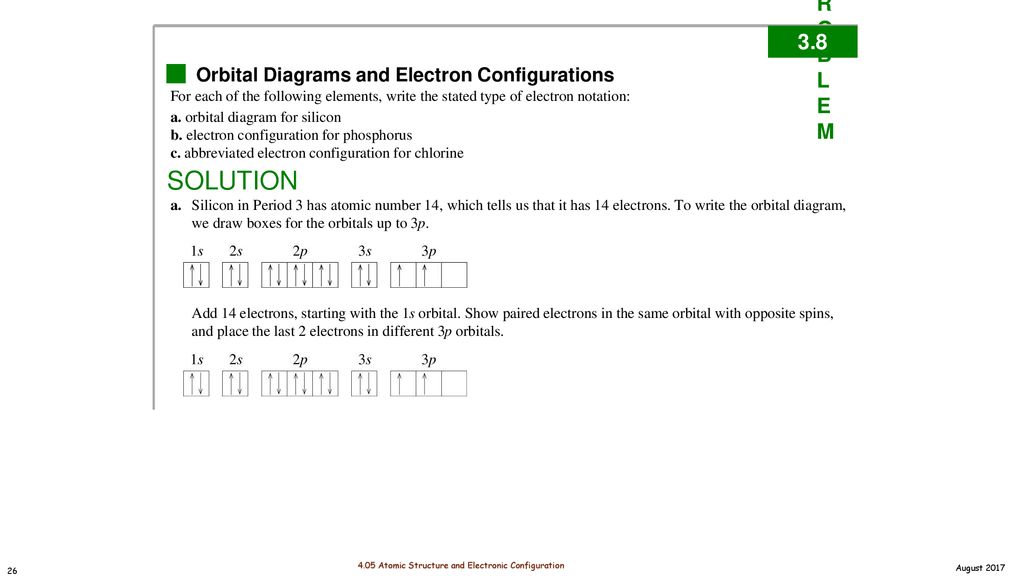
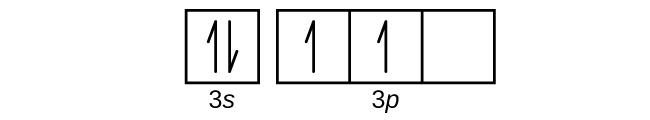
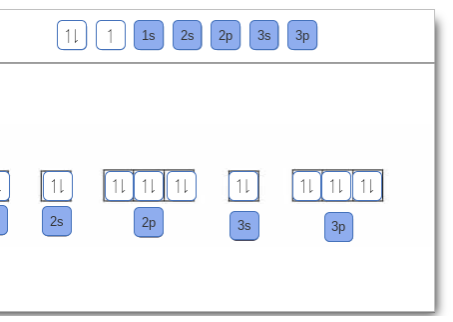
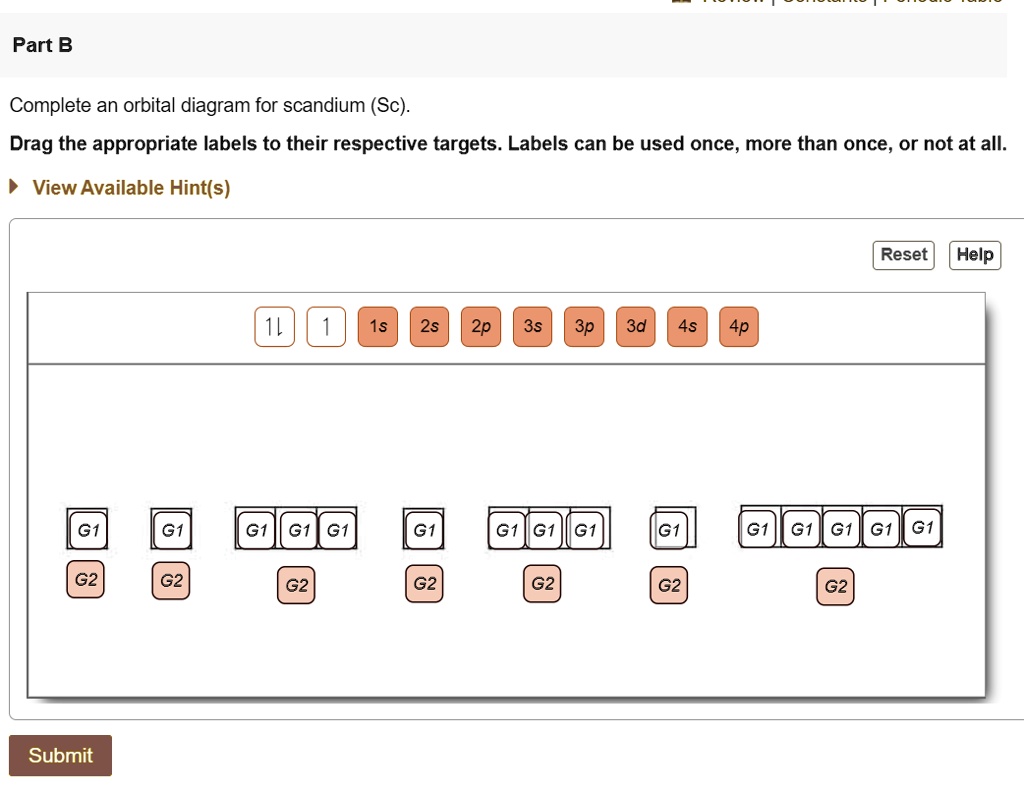
Complete An Orbital Diagram For Scandium (sc). Answer to Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. 3d. 4p. Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation). 1. scandium. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. Contrary to what you may have seen, for Sc and the remaining elements ... Complete An Orbital Diagram For Scandium (Sc), Scandium (Sc) Feb 10, 2021 · For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 . So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. You are watching: Complete an orbital diagram for scandium
Draw An Orbital Diagram For Scandium (sc) Scandium (Sc) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Contrary to what you may have seen, for Sc and the remaining elements, the 4s is not lower in energy than the 3d. In fact, for elements with.An orbital diagram is similar to electron configuration, except that ...
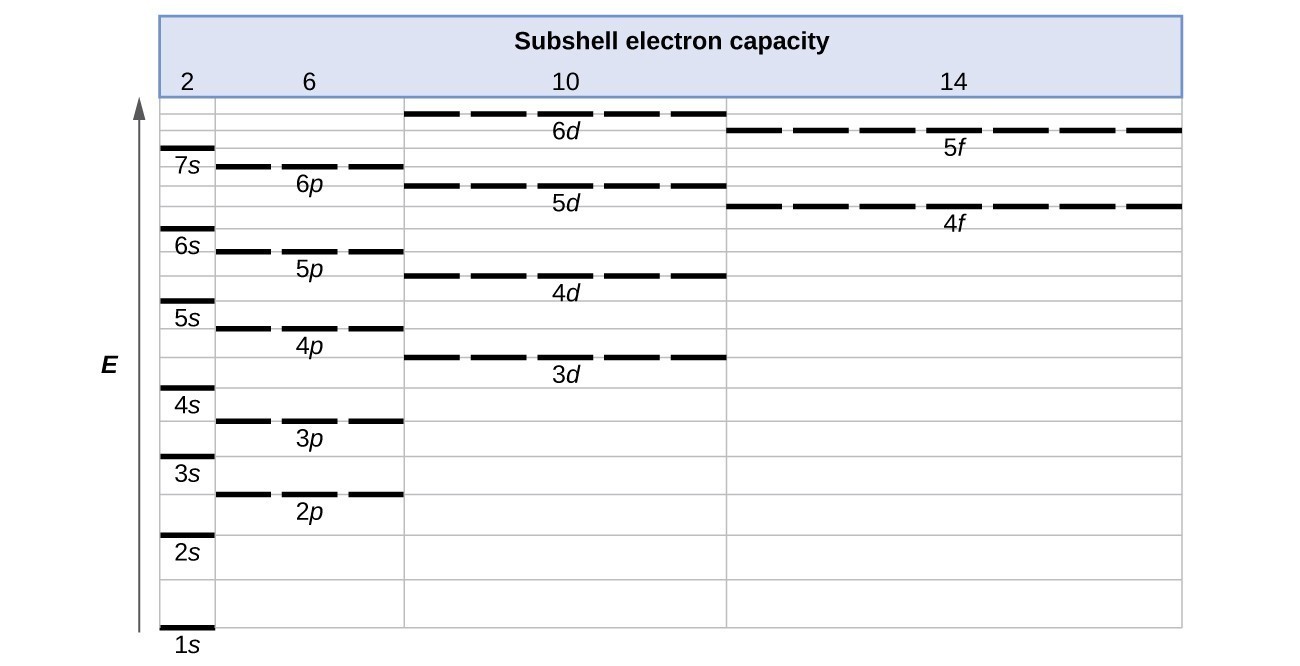
Orbital diagram for scandium sc
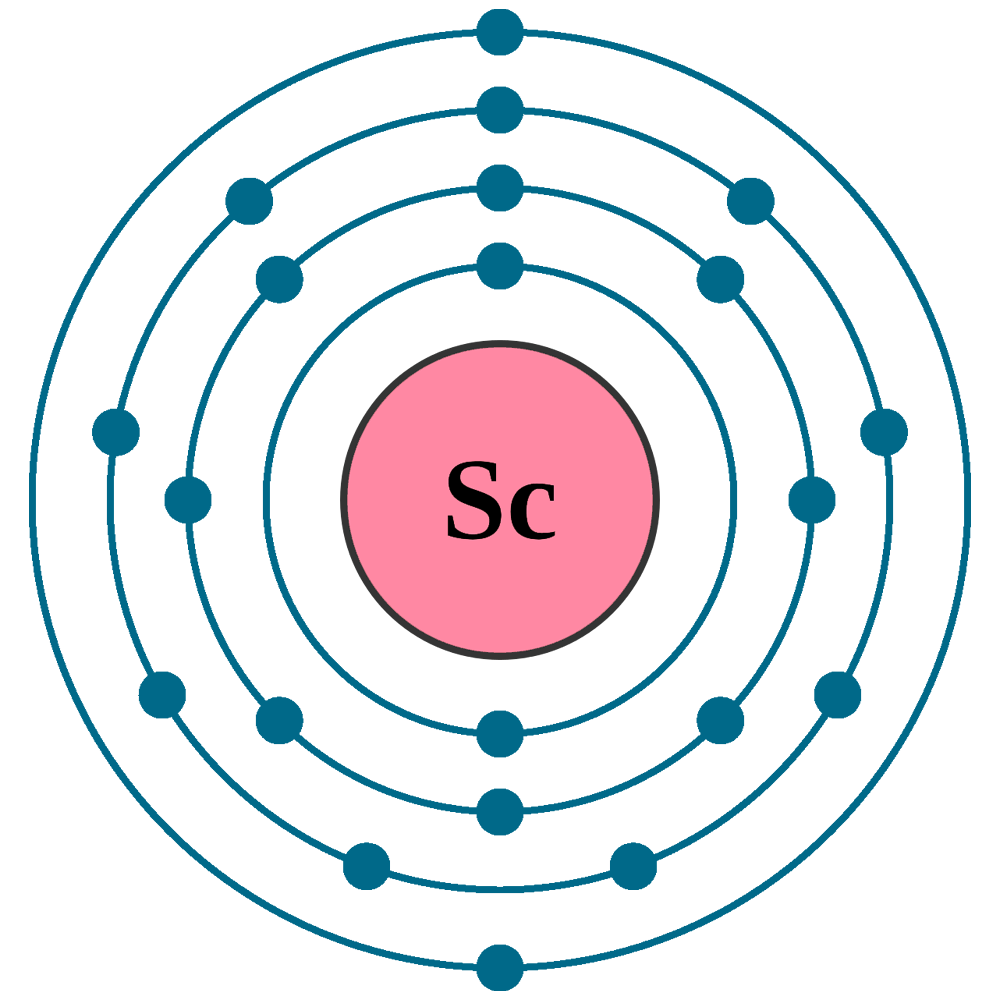
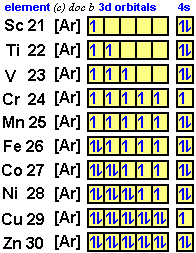
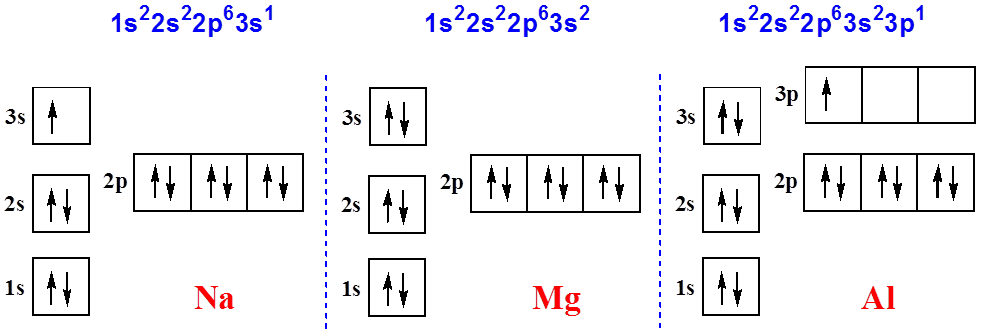
Scandium(Sc) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell... Complete An Orbital Diagram For Scandium (sc). Nov 09, 2018 · An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ... Draw An Orbital Diagram For Scandium (sc) Oct 10, 2018 · Use this tool to draw the orbital diagram. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1. So for scandium the 1st and 2nd electron must be.Question ...
Orbital diagram for scandium sc. Draw An Orbital Diagram For Scandium (sc) Oct 10, 2018 · Use this tool to draw the orbital diagram. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1. So for scandium the 1st and 2nd electron must be.Question ... Complete An Orbital Diagram For Scandium (sc). Nov 09, 2018 · An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ... Scandium(Sc) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell...






/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)














0 Response to "39 orbital diagram for scandium sc"
Post a Comment