39 solid liquid phase diagram
PDF Phase Diagrams, Solid Solutions, Phase Transformations Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%). Phase Diagram for Water | Chemistry for Non-Majors Notice one key difference between the general phase diagram and the phase diagram for water. In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance in that its solid state is less dense than the liquid state. Ice floats in liquid water.
PDF Experiment 1 Solid - Liquid Phase Diagram The binary solid-liquid phase diagram for the naphthalene-diphenylamine system will be constructed from cooling curves. Several mixtures of different ratios of the two components will be melted, and temperature versus time curves will be plotted as the mixtures cool.
Solid liquid phase diagram
Phase Diagrams | Liquids and Solids - Nigerian Scholars A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. Binary Solid-Liquid Phase Diagram - ENG 5304 - Foundations ... In this lab, a solid-liquid phase diagram was constructed using several cooling curves of a. mixture at different compositions. The arrest and breaks of the cooling curves were used to. determine the temperatures for the points at each composition. The phase diagram was used to. determine the eutectic point of the mixture. PDF Determination of the Solid-Liquid Phase Diagram for ... • Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ...
Solid liquid phase diagram. › files › matter_solidChem4Kids.com: Matter: Solids Solid Basics What is one physical characteristic of a solid? Solids can be hard like a rock, soft like fur, a big rock like an asteroid, or small rocks like grains of sand. The key is that solids hold their shape and they don't flow like a liquid. A rock will always look like a rock unless something happens to it. The same goes for a diamond. PDF Simple Solid - Liquid Phase Equilibria An understanding of solid liquid phase equilibria is required to describe and calculate phase diagrams of interest in both fundamental materila science and applications such as crystallizer design. A very simple phase diagram is shown below. For this system the two solid phase do not mix (form intercallated crystals) or form solid compounds. Liquid and Solid Solution phase changes - First Year ... The solid/liquid solution phase diagram can be quite simple in some cases and quite complicated in others. Let's begin by looking at a simple two-component phase diagram with components that are fully miscible in both the liquid and solid phase. The diagram to the right shows a simple two-component A,B system solid-liquid phase diagram. › metallurgy › metalsSolid Solution of Metals: With Diagram | Metallurgy If the mutual solid solubility is restricted (as in Cu-Ag system) to only those portions of the phase diagram that are linked to the pure elements, the solid solutions formed are called as primary (or terminal) solid solutions, which have same crystal structure as of solvent metals.
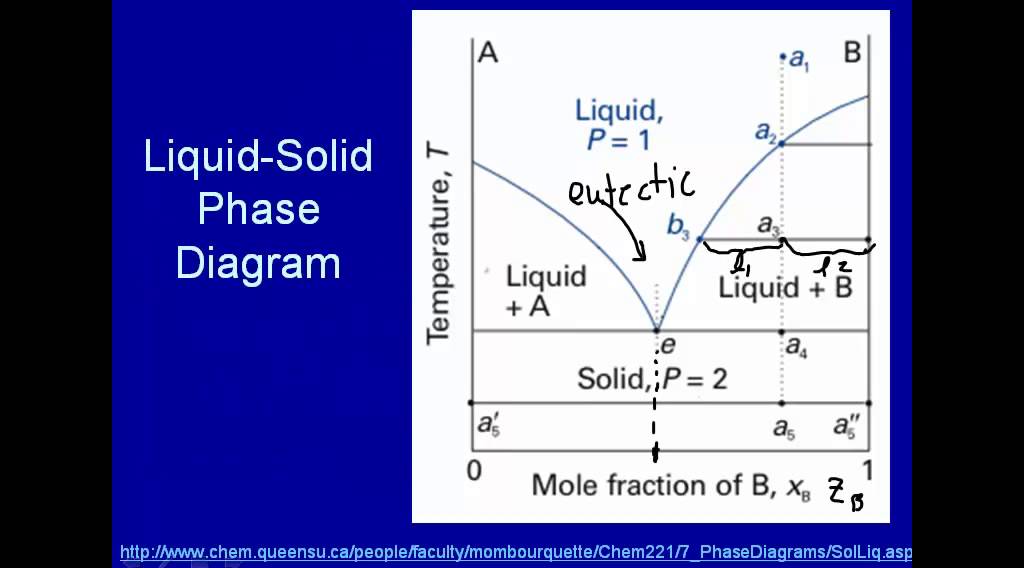
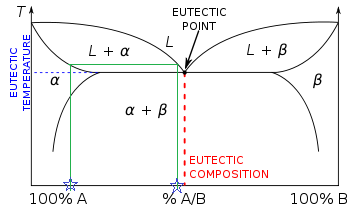
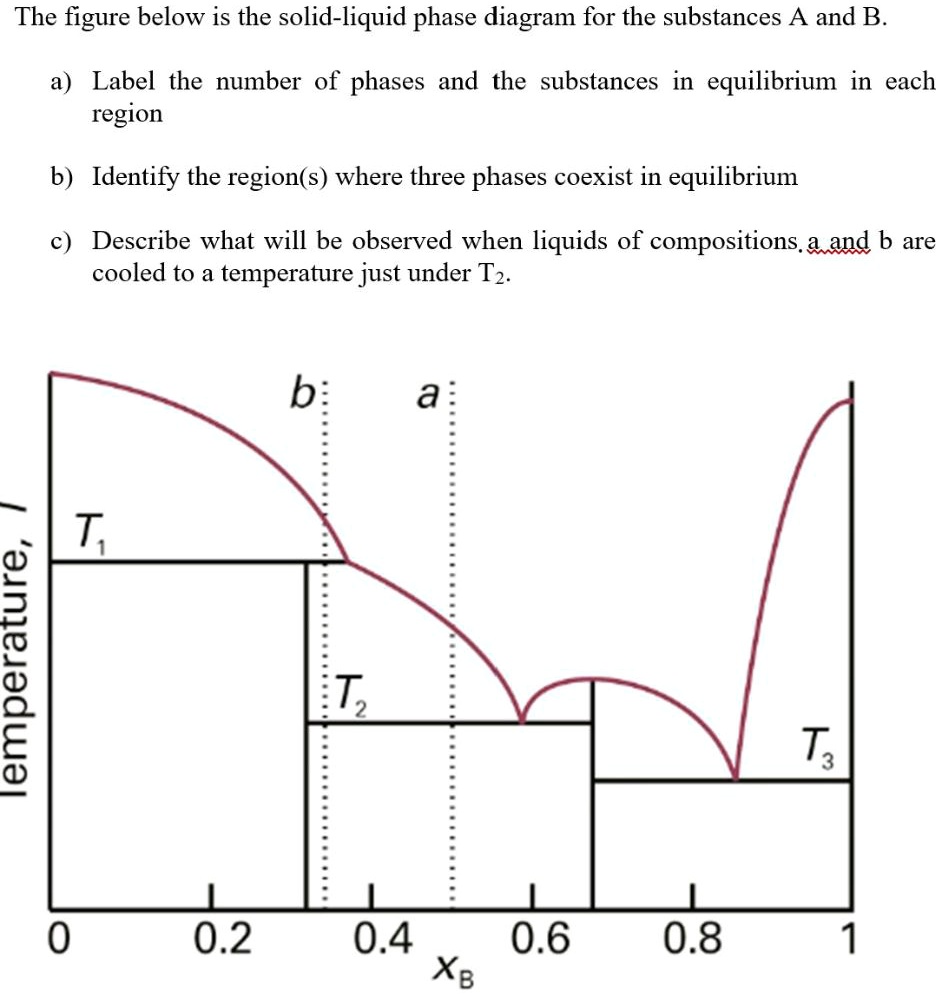
“Bligh and Dyer” and Folch Methods for Solid–Liquid ... 27.3.2017 · The first step is a solid–liquid extraction from an initial amorphous gel state, followed by a second liquid–liquid partitioning step. In the second step, the initially extracted mixture of all biomolecules is separated into two phases: an organic-rich phase (OR) containing the total lipids and a water-rich phase (WR) containing others compounds (sugars, proteins, etc.). LAS 3_LESSON ACTIVITY SHEET IN GENERAL CHEMISTRY II.docx ... on the melting point of the solid. Anywhere on this line, there is equilibrium between the solid and the liquid. 2. The blue line divides the liquid and gas phases, and represents vaporization (liquid to gas) and condensation (gas to liquid) points. Vaporization (or condensation) curve - the curve on a phase diagram which represents the transition between gaseous and liquid states. EUTECTICS - A Practical Guide to Phase Diagrams Below is the phase diagram of the lead (Pb)-tin (Sn) system. In this section, the Pb-Sn phase diagram will be discussed in detail, but first there a few basic components of phase diagrams to discuss. First there are the axes. The vertical axis shows temperature, while the horizontal axis shows the concentration of some species. Chapter 8 Phase Diagrams - Central Michigan University The system enters the two phase region labeled 'liquid + B'. Pure solid B begins to come out of solution and the remaining liquid becomes richer in A. (2) a 2 ® a 3. More of the solid forms, and the relative amounts of the solid and liquid (which are in equilibrium) are given by the lever rule.
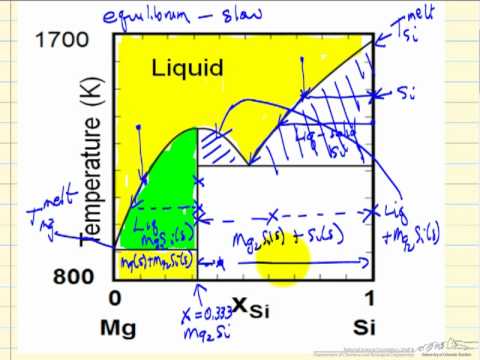
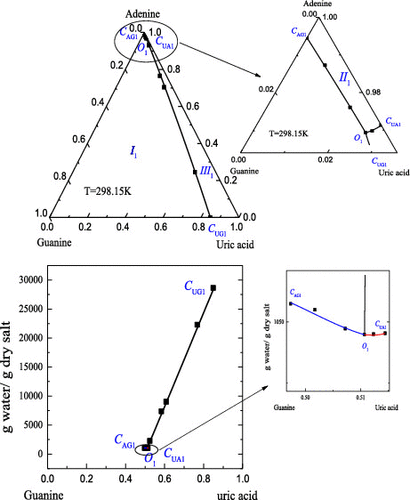
Solid-Liquid Phase Diagrams - YouTube Describes the regions of a liquid-solid, T-x phase diagram for a system composed of Mg and Si. Made by faculty at the University of Colorado Boulder Departme... PDF EXPERIMENT 4 - Constructing a Solid-Liquid Phase Diagram ... Solid-Liquid Phase Diagram Using Di erential Scanning Calorimetry Objective The purpose of this laboratory is to use a modern di erential scanning calorimeter to measure the liquid-solid phase diagram (T-˜) and associated thermodynamic parameters (eutectic point, H, C p, S, etc..) of a binary compound or elemental system. Introduction chem.libretexts.org › Phase_DiagramsPhase Diagrams - Chemistry LibreTexts May 03, 2021 · The solid liquid line is "normal" (meaning positive sloping). For this, complete the following: 1. Roughly sketch the phase diagram, using units of atmosphere and Kelvin. Answer. 1-solid, 2-liquid, 3-gas, 4-supercritical fluid, point O-triple point, C-critical point -78.5 °C (The phase of dry ice changes from solid to gas at -78.5 °C) 2. Solid-Liquid Phase Equilibrium and Diagram of a l ... The equilibrium solubility data of l-phenylalanine (C9H11NO2) dissolved in hydrogen peroxide solutions with different concentrations were obtained at 273.15 and 283.15 K by the use of a static method under ambient pressure (p = 101.2 kPa), and the ternary phase diagrams of the C9H11NO2-H2O2-H2O system were constructed according to the determined solubility. At 273.15 and 283.15 K, two neat ...
PDF Solid - Liquid Phase Diagram of a Binary Mixture: The ... Solid - Liquid Phase Diagram of a Binary Mixture: The Question of Fatty Acids Dimers in the Liquid Phase Gary L. Bertrand University of Missouri-Rolla Overview Two components will be assigned for this experiment. One will be a carboxylic acid, and the other will be a relatively non-polar material.
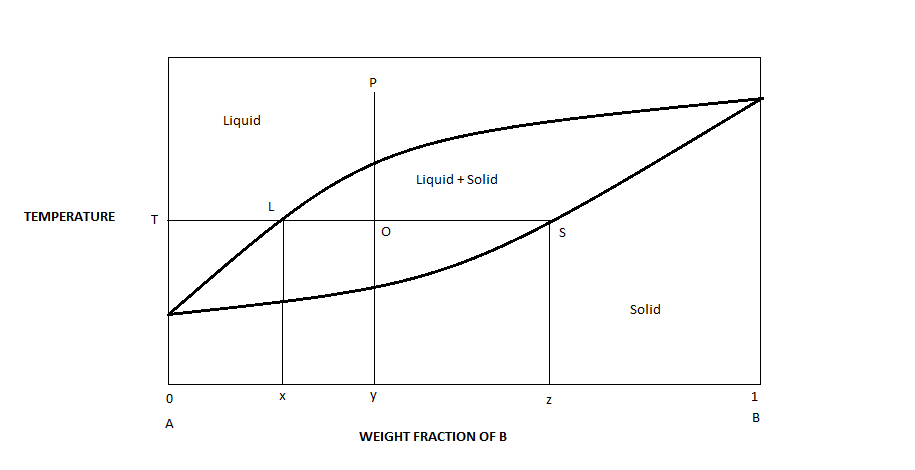
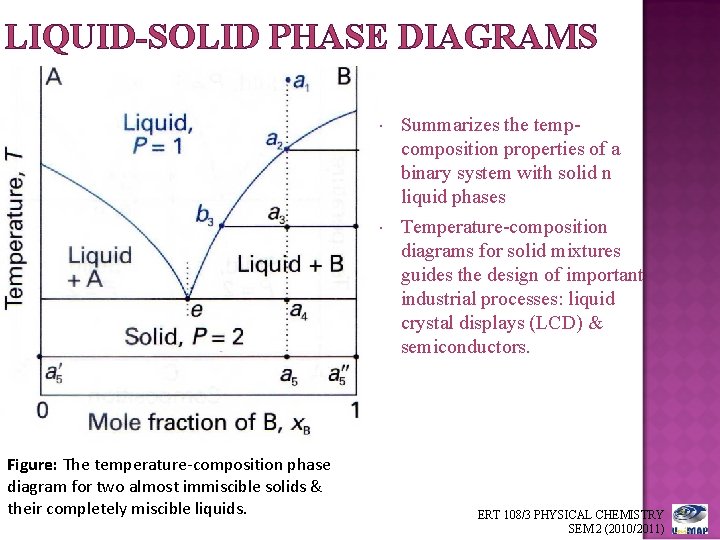
PDF Chapter Outline: Phase Diagrams Isomorphous system -complete solid solubility of the two components (both in the liquid and solid phases). Binary Isomorphous Systems (I) Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidusline separates solid from liquid + solid α+ L α
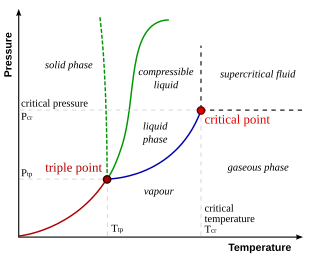
Phase Diagrams States of Matter and Phase Changes Terminology of Phase Diagrams Critical Temperature and Critical Pressure This is the point on the phase diagram above which a liquid is unable to form. It cannot form because the particles have TOO much kinetic energy to form intermolecular bonds needed for liquids.
How to figure out which phase on a phase diagram will be ... If it is decreasing, the liquid phase is denser, if it is increasing, the solid phase is denser. For example, the phase diagram of water has a negative solid-liquid line; the liquid phase of water is denser. The densest phase exists at the highest pressure and lowest temperature. This comes back to looking at the top left corner of the graph. Share
Phase Diagrams | Boundless Chemistry - Lumen Learning Phase diagrams are divided into three single phase regions that cover the pressure-temperature space over which the matter being evaluated exists: liquid, gaseous, and solid states. The lines that separate these single phase regions are known as phase boundaries.
fractory.com › iron-carbon-phase-diagramIron-Carbon Phase Diagram Explained [with Graphs] - Fractory Mar 10, 2020 · It will form a solid solution with α, γ, and δ phases of iron. Types of Ferrous Alloys on the Phase Diagram. The weight percentage scale on the X-axis of the iron carbon phase diagram goes from 0% up to 6.67% Carbon. Up to a maximum carbon content of 0.008% weight of Carbon, the metal is simply called iron or pure iron.
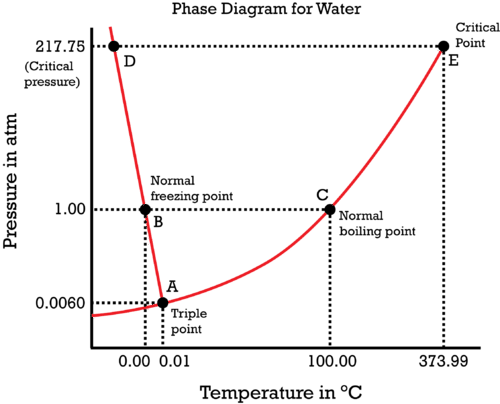
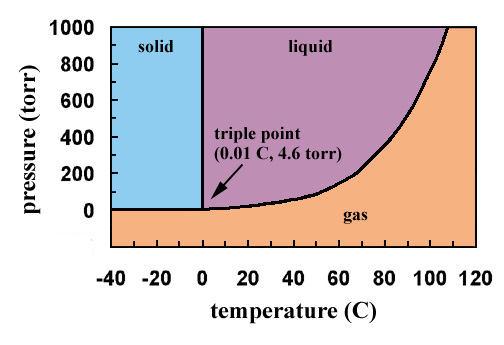
Phase Diagrams - Chemistry The temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Phase diagrams are combined plots of three pressure-temperature equilibrium curves: solid-liquid, liquid-gas, and solid-gas.
T&HE: LESSON - 19 p-V DIAGRAM, T-s DIAGRAM, p-T DIAGRAM ... 19.1. p-V DIAGRAM. 19.1.1. p-V diagram for water (solid-liquid-vapor region) If we heat ice at different vapor pressures and note down the corresponding change in volumes, the saturation state points for solid, liquid and vapor (state from which a change of phase may occur without change of pressure and temperature) for different pressures may be obtained on a p-V diagram.
Phase (matter) - Wikipedia An unusual feature of the water phase diagram is that the solid–liquid phase line (illustrated by the dotted green line) has a negative slope. For most substances, the slope is positive as exemplified by the dark green line. This unusual feature of water is related to ice having a lower density than liquid water.
Solid-liquid Phase Diagrams: Tin and Lead solid-liquid phase diagrams: tin and lead This page explains the relationship between the cooling curves for liquid mixtures of tin and lead, and the resulting phase diagram. It also offers a simple introduction to the idea of a eutectic mixture.
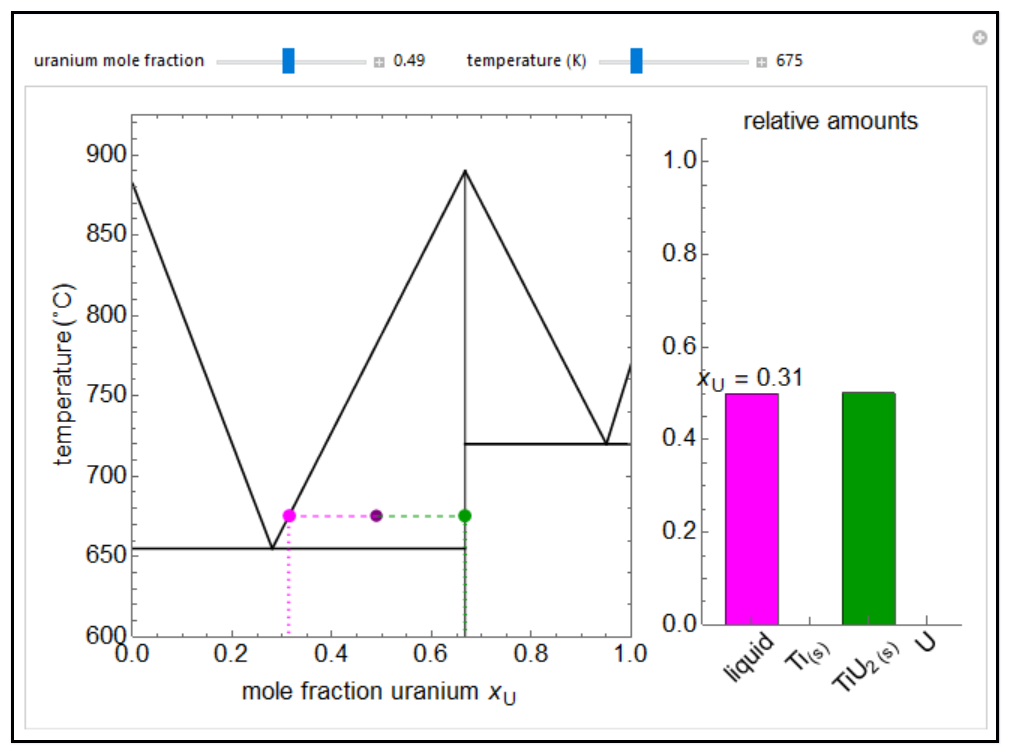
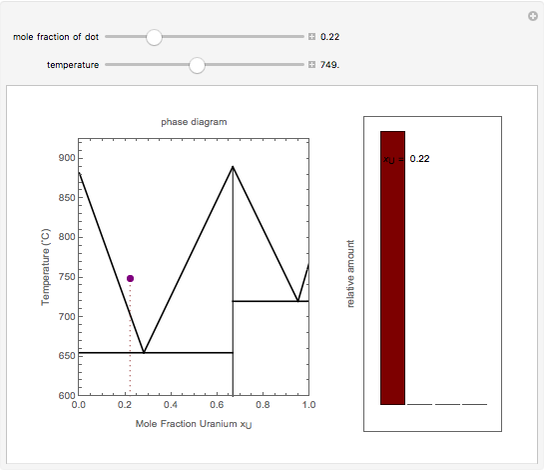
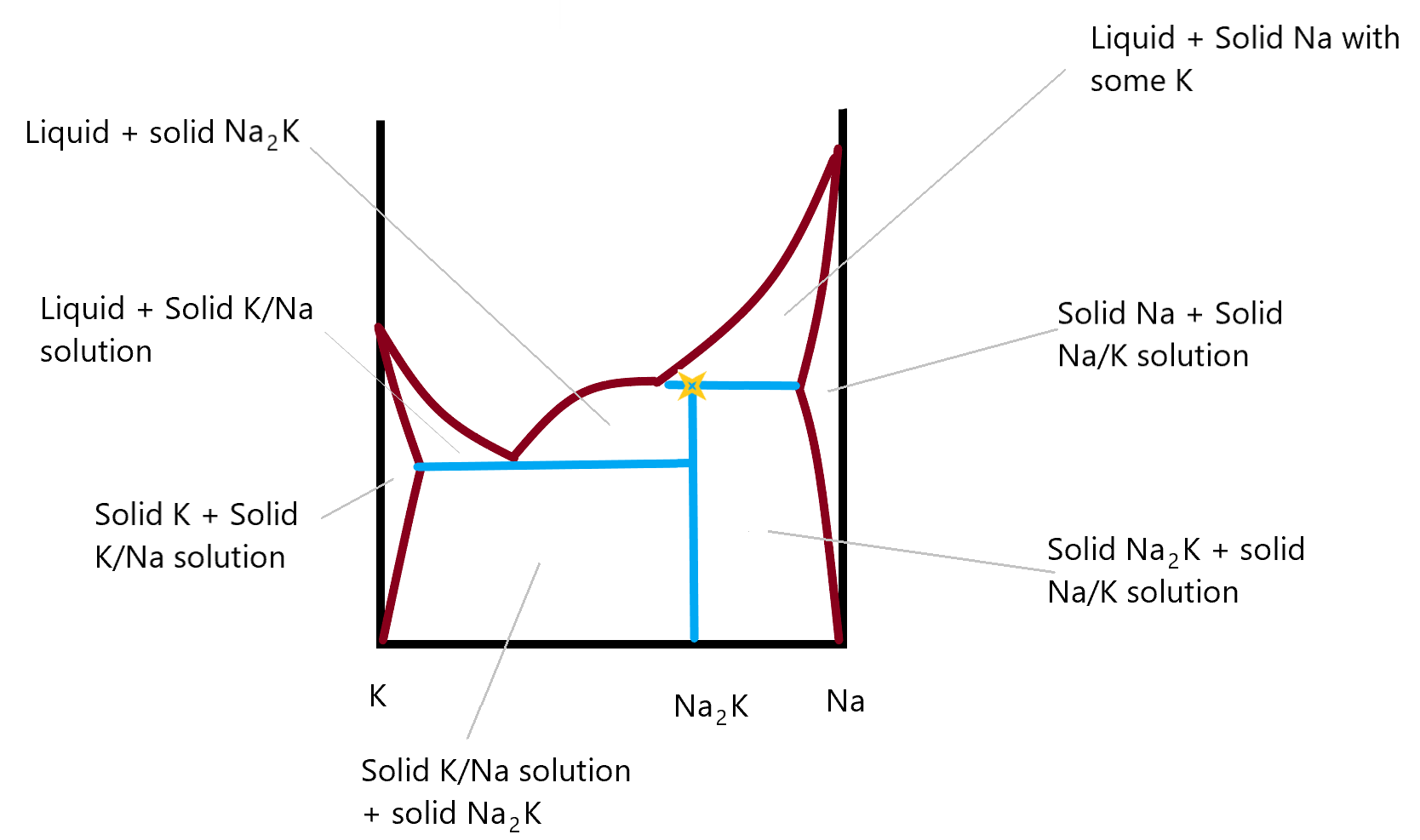
Solid-Solid-Liquid Phase Diagram (Interactive Simulation ... Describes an interactive simulation that shows the phase diagram (temperature versus mole fraction) for two solids that form a solid compound. Heat can be ad...
Solid Liquid Phase Diagram - Solid-Liquid Phase Diagram in ... Solid-Liquid Phase Diagram in a Two-Component System 1 Introduction The substances we encounter everyday are commonly mixtures of two or more components. For example, brass is a mixture of copper and zinc, and dish-washing detergent is a mixture of many chemicals. The components may interact with each other in a variety of different manners.
Binary Solid-Liquid Phase Diagram | Chem Lab Introduction Solid-liquid phase diagrams show the phase relationships in mixtures of two or more components and are very important in understanding the behavior of mixtures in metallurgy, material science and geology.
1.14 Phase Diagrams Quiz Flashcards | Quizlet For a given sample of carbon dioxide (CO2) you increase the temperature from −80°C to 0°C and decrease the pressure from 30 atm to 5 atm . On a phase diagram this causes a crossing of both the solid-liquid boundary and the liquid-gas boundary.
PDF Binary Solid-Liquid Phase Diagram Introduction remaining liquid solution. In the salt water analogy, the solid ice (pure H2O) is in equilibrium with the liquid H2O that remains in the unfrozen salt water. Mixtures of naphthalene and diphenylamine, both solids in the pure state at room temperature, will be prepared and their phase transitions studied by means of a thermal analysis.
Solid-liquid Phase Diagram | USP Technologies Solid-liquid Phase Diagram . Ref: P.A. Giguere. "Complements au Nouveau Traite de C. himie Minerale - No. 4 - Peroxyde d'Hydrogene et Polyoxydes d'Hydrogene" Paris, Masson 1975 (181 p).
PDF Liquid-Solid Phase Diagrams - Texas A&M University Liquid-Solid Phase Diagrams Solid and liquid phases can be present below the boiling point (e.g., immiscible pair of metals right up to their melting points (As and Bi) 2-component liquid at temperature a1: (1) a1 6 a2 System enters "Liquid+B" pure solid B comes out of solution, remaining liquid richer in A (2) a2 6 a3 More solid B forms, equal
10.4 Phase Diagrams - Chemistry Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
chemdictionary.org › phase-diagramPhase Diagram | Explanation, Definition, Summary & Facts Oct 14, 2019 · Water phase diagram. The phase diagram of water is very unique in a sense that boundary which separates the solid phase (ice) and liquid phase bend towards the left side as compared to other substance, where it bends towards right side. The reason of left bend of solid-liquid phase boundary is because ice has low density as compared to liquid ...
Solid Solution Phase Diagram - James Madison University The solid solution phase diagram explains the behavior of chemical solid solution series, such as the transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase, or the transition from high temperature magnesium-rich to low temperature iron-rich crystals in ferromagnesium minerals (e.g. olivine, pyroxene).
Template for triangular diagrams in MS Excel : Phasediagram Vapor-liquid equilibrium, solid-liquid equilibrium, liquid-liquid equilibrium and others. The data for the three components can be given in mol percent or mass percent. The template was used for making the triangular diagram to the right and for diagrams on several other pages such as the phase diagrams for the mixed solvent systems.
Phase Diagram | Explanation, Definition, Summary & Facts 14.10.2019 · Water phase diagram. The phase diagram of water is very unique in a sense that boundary which separates the solid phase (ice) and liquid phase bend towards the left side as compared to other substance, where it bends towards right side. The reason of left bend of solid-liquid phase boundary is because ice has low density as compared to liquid ...
Iron-Carbon Phase Diagram Explained [with Graphs] - Fractory 10.3.2020 · It will form a solid solution with α, γ, and δ phases of iron. Types of Ferrous Alloys on the Phase Diagram. The weight percentage scale on the X-axis of the iron carbon phase diagram goes from 0% up to 6.67% Carbon. Up to a maximum carbon content of 0.008% weight of Carbon, the metal is simply called iron or pure iron.
Solid-liquid phase equilibrium and phase diagram of the ... The phase diagram belongs to a simple-type ternary system, and neither double salt nor solid solution was formed. Based on the phase diagrams of this system at 298.15 and 338.15 K, the cycle separation process for CsNO 3 recovery from the eluant of sodium nitrate mixture solution was evolved.
PDF Determination of the Solid-Liquid Phase Diagram for ... • Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ...
Binary Solid-Liquid Phase Diagram - ENG 5304 - Foundations ... In this lab, a solid-liquid phase diagram was constructed using several cooling curves of a. mixture at different compositions. The arrest and breaks of the cooling curves were used to. determine the temperatures for the points at each composition. The phase diagram was used to. determine the eutectic point of the mixture.
Phase Diagrams | Liquids and Solids - Nigerian Scholars A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance.

















0 Response to "39 solid liquid phase diagram"
Post a Comment