40 mo diagram for h2
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine PDF MO Diagrams for Linear and Bent Molecules Build MO diagram. We expect six MOs, with the O 2pytotally nonbonding. Water H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -13.6 eV -15.8 eV -32.4 eV Based on the large ΔE, we expect O 2s to be almost nonbonding. Water With the orbital shapes, symmetries, and energies in hand we can make the MO ...
N2+ Mo Diagram - schematron.org The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.

Mo diagram for h2
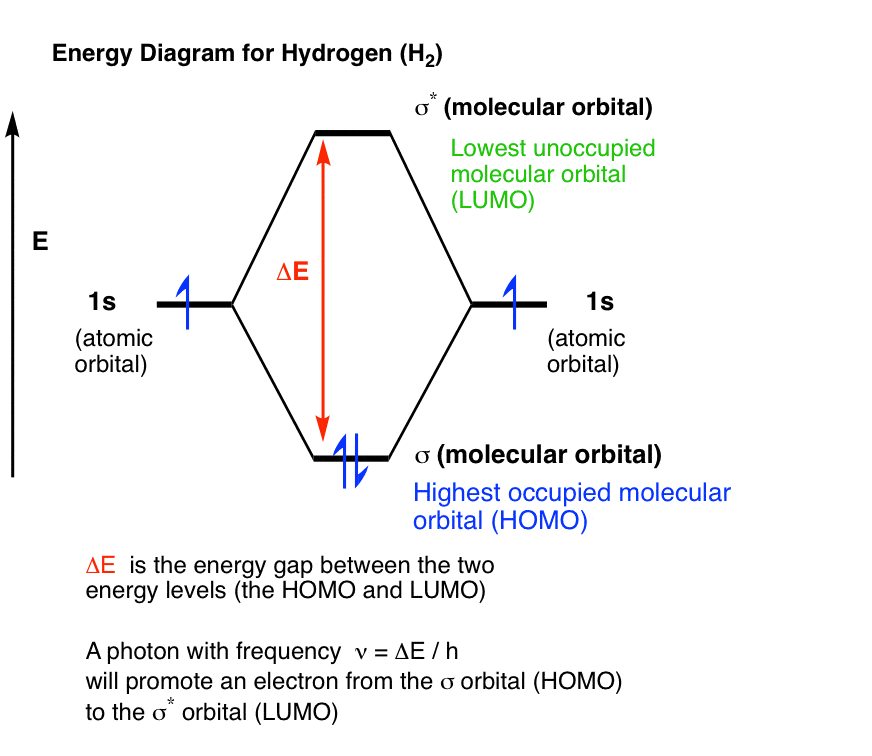
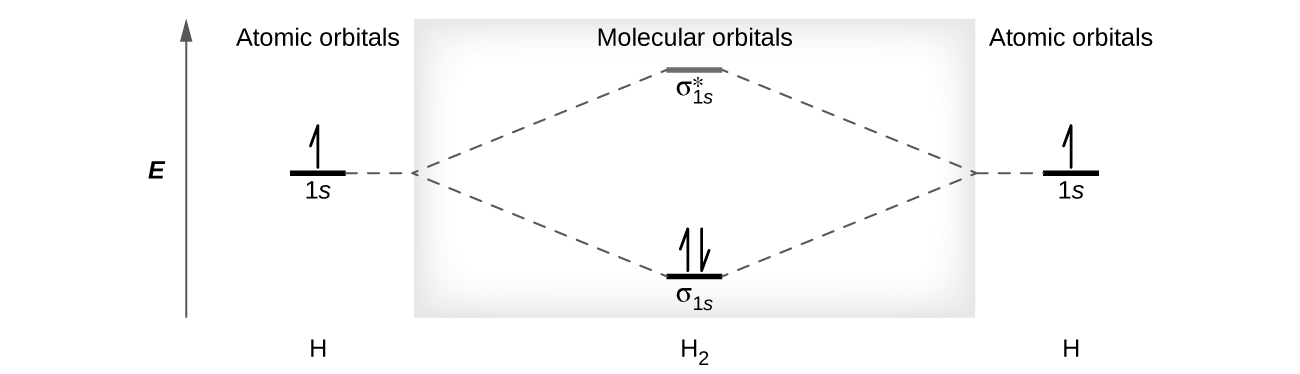
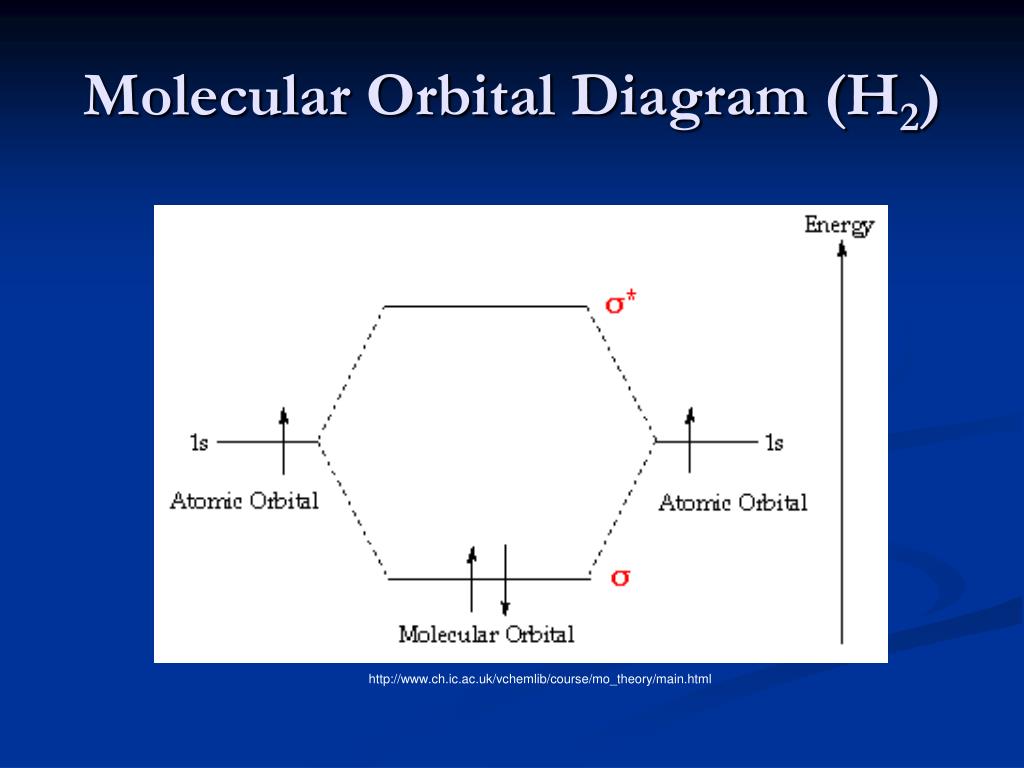
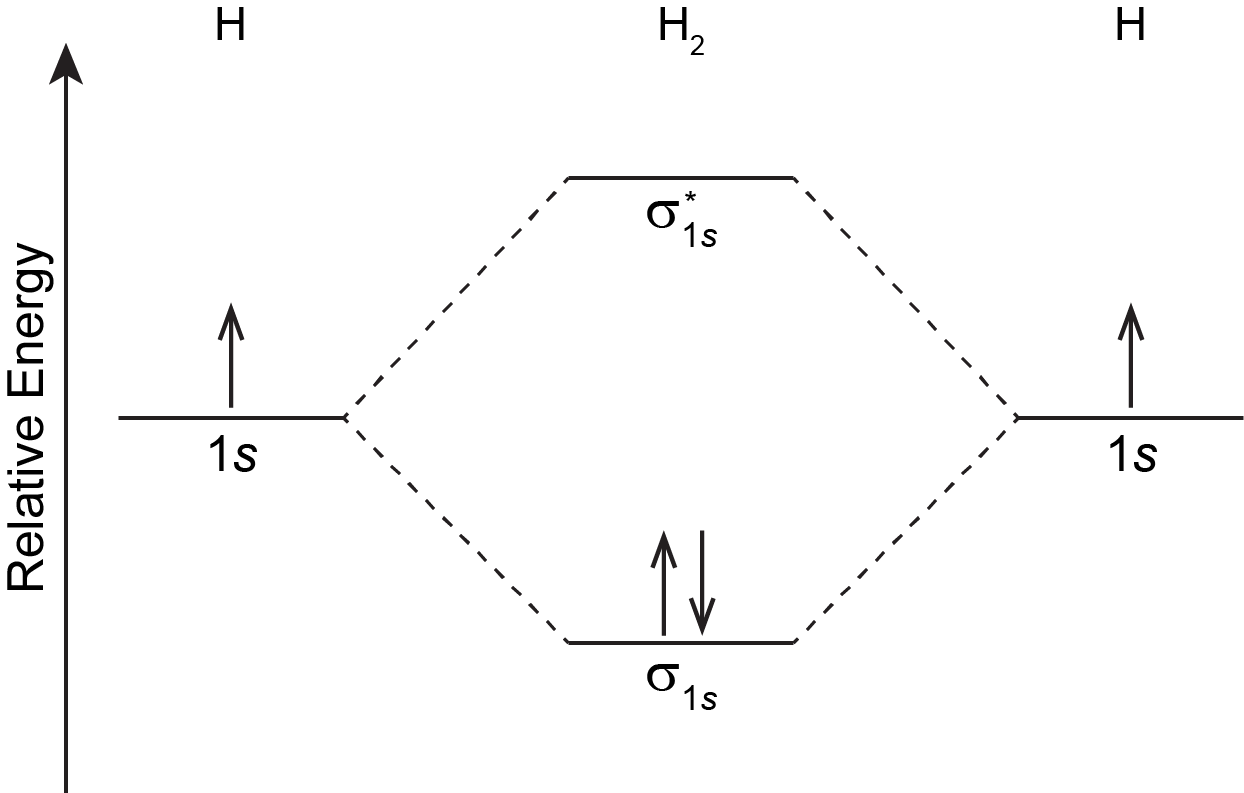
How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals. Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. EOF
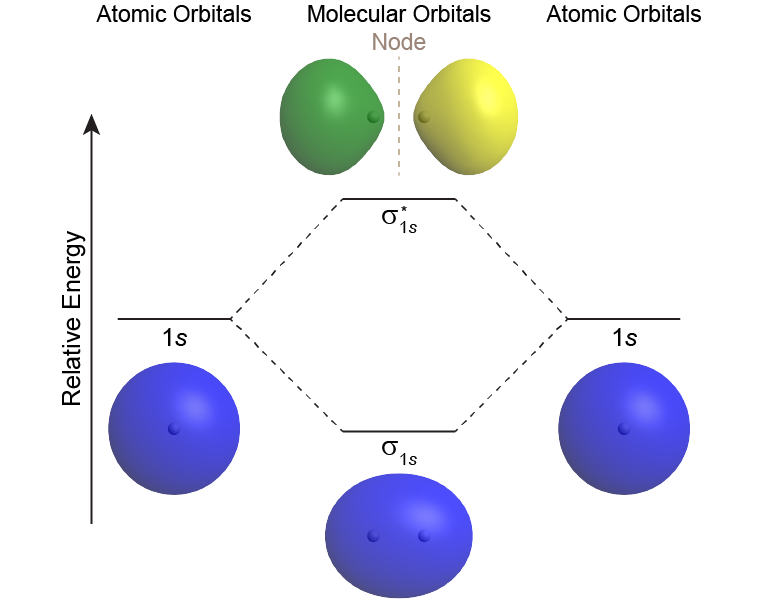
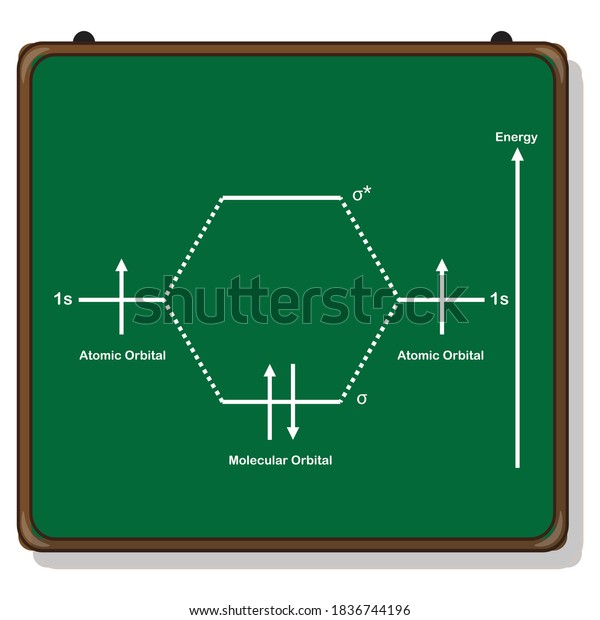
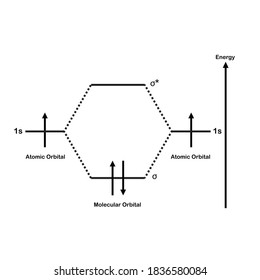
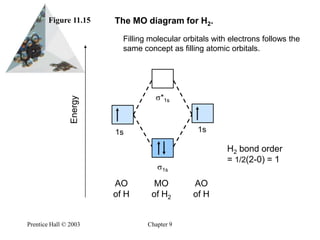
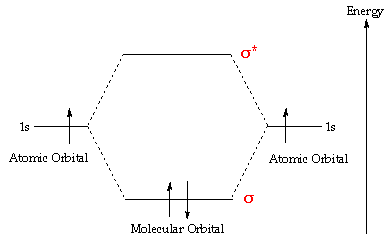
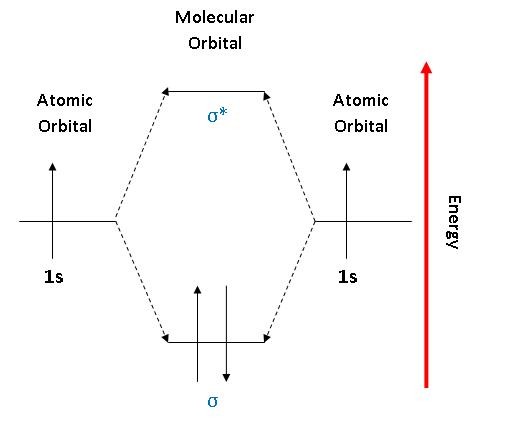
Mo diagram for h2. CB VII Molecular Orbital (MO) Theory theory; resulting in better info on: bond energy bond order magnetic properties of molecules ... Application to H2 Molecule ... Fig.11.20 MO Theory for H2.8 pages inorganic chemistry - MO diagram of BeH₂ - Chemistry Stack ... $$\ce{Be + H2 <=> BeH2}$$ The MO for $\ce{H2}$, which is shown in the figure below is taken from Wikipedia. The right side of the diagram you showed neither represents a hydrogen molecule, nor two independent (and hence equivalent) hydrogen atoms. Construct The Molecular Orbital Diagram For H2 And Then ... on Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. Addition of two orbitals can lead to bonding MO and anti-bonding MO. On this page MO for schematron.org Figure1: MO diagram for H2. The filling of Bond order = 1/2 (#e- in bonding MO - #e- in antibonding MO). For H2, bond. one electron in bonding orbital; so ... Molecular Orbital Diagram For He2+ - schematron.org on Molecular Orbital Diagram For He2+. He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s. This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s. The bond order of a simple molecule can be determined by looking at the number of electrons in bonding and antibonding molecular orbitals. Like electrons in.
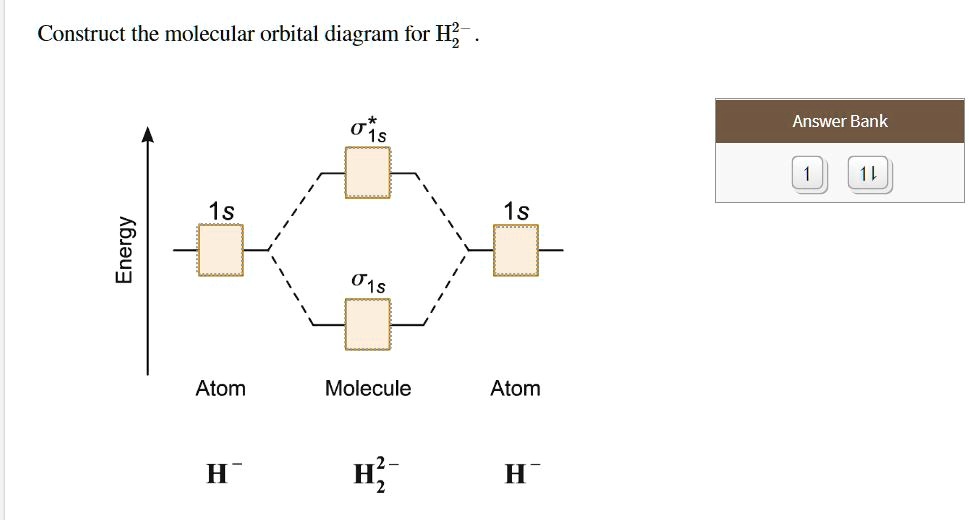
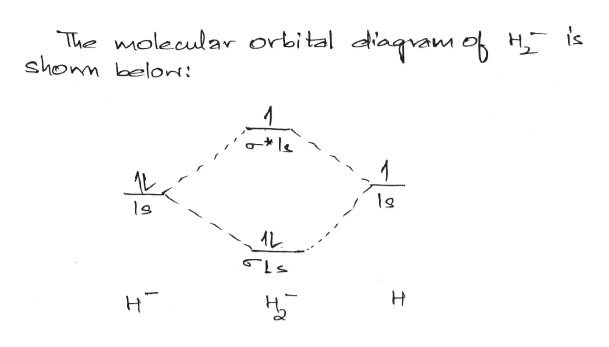
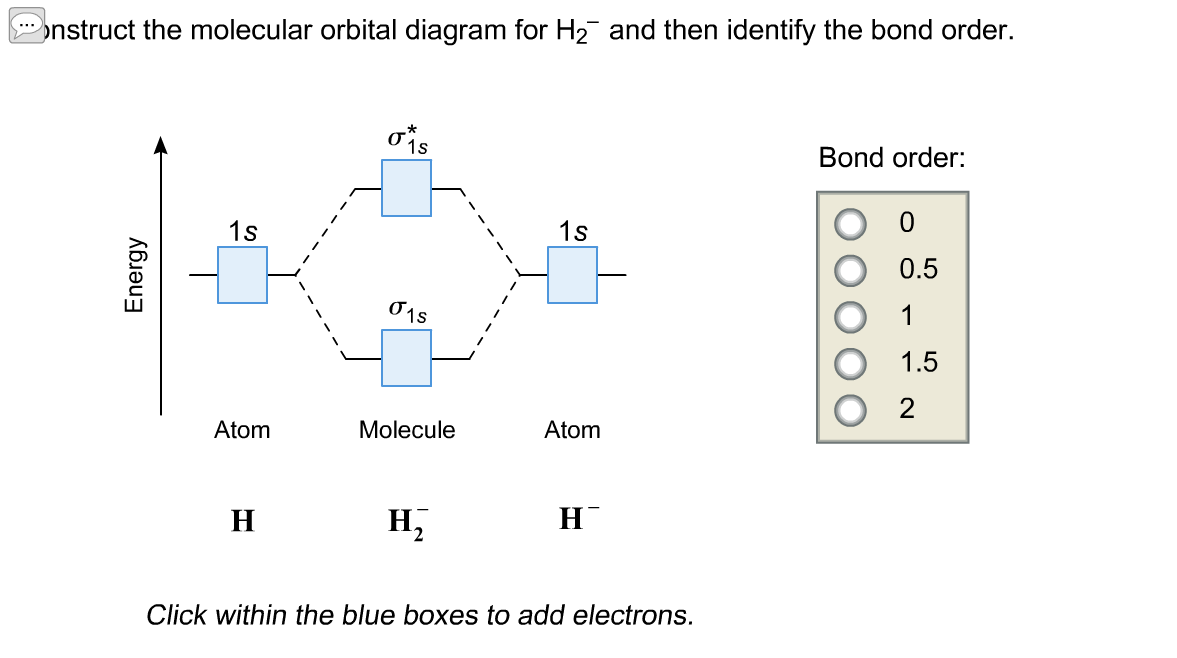
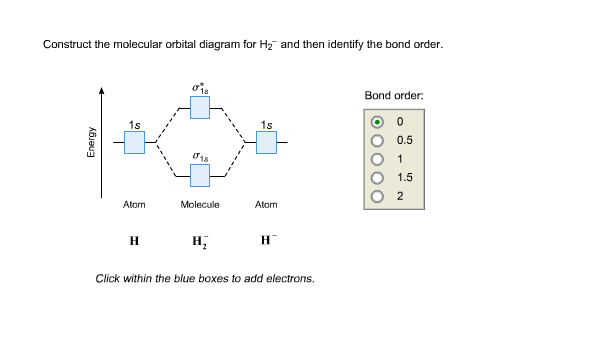
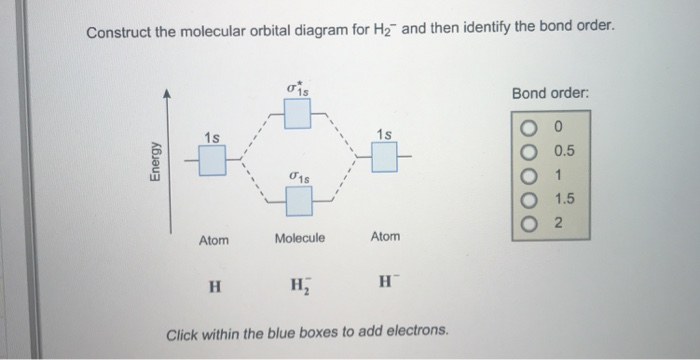
Complete An Mo Energy Diagram For H2+. 1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is . Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ... chemical bonding - Molecular orbitals of H2 and He2 ... Now consider the structure of N 2.There are 2 × 5 = 10 valence electrons to accommodate. These electrons occupy the five lowest-energy MOs and hence result in the configuration 1σ 2 2σ 2 1π 4 3σ 2.Note that only the orbitals in the lower portion of the diagram of Figure 14 are occupied. This configuration accounts for the considerable strength of the bonding in N 2 and consequently its ... PDF Molecular Orbital (MO) Theory of the H2 molecule Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is given by: gs ψψ αβ ... Qualitative MO theory orbital diagram for homonuclear diatomics composed of 1st or 2nd row elements:
Complete An Mo Energy Diagram For H2+. - schematron.org 1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. σ bonding MO that is lower in energy than the constituent 1s AOs and an antibonding σ* MO that is at a higher energy than the 1s AOs. [1] EOF Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.



















0 Response to "40 mo diagram for h2"
Post a Comment