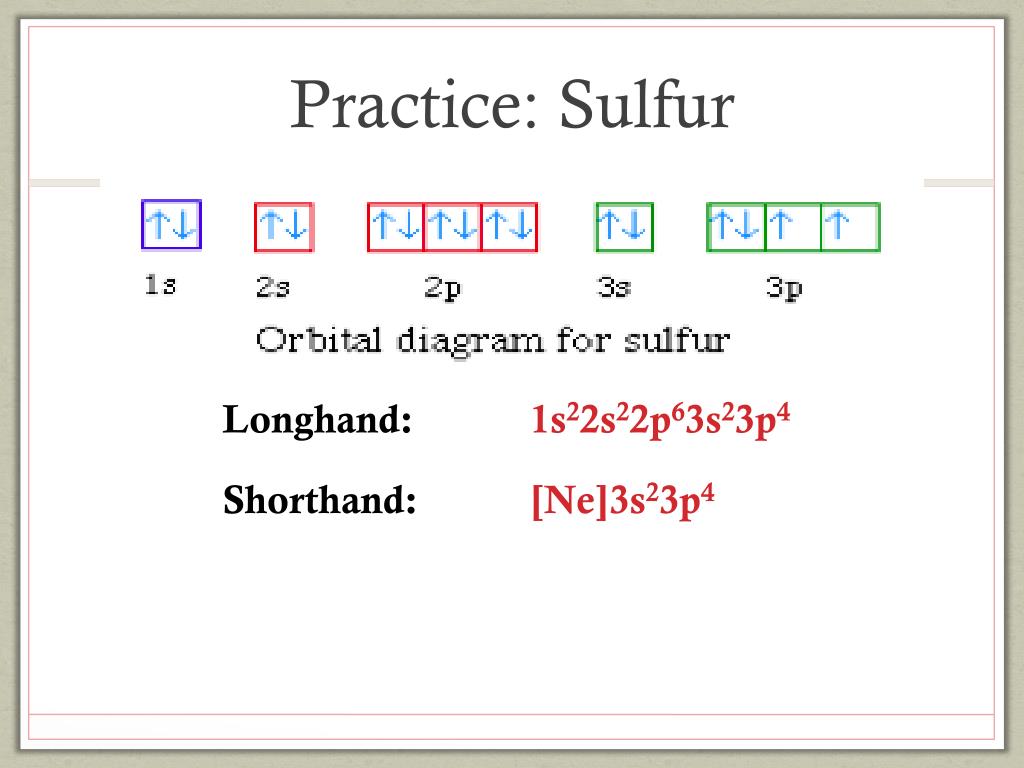
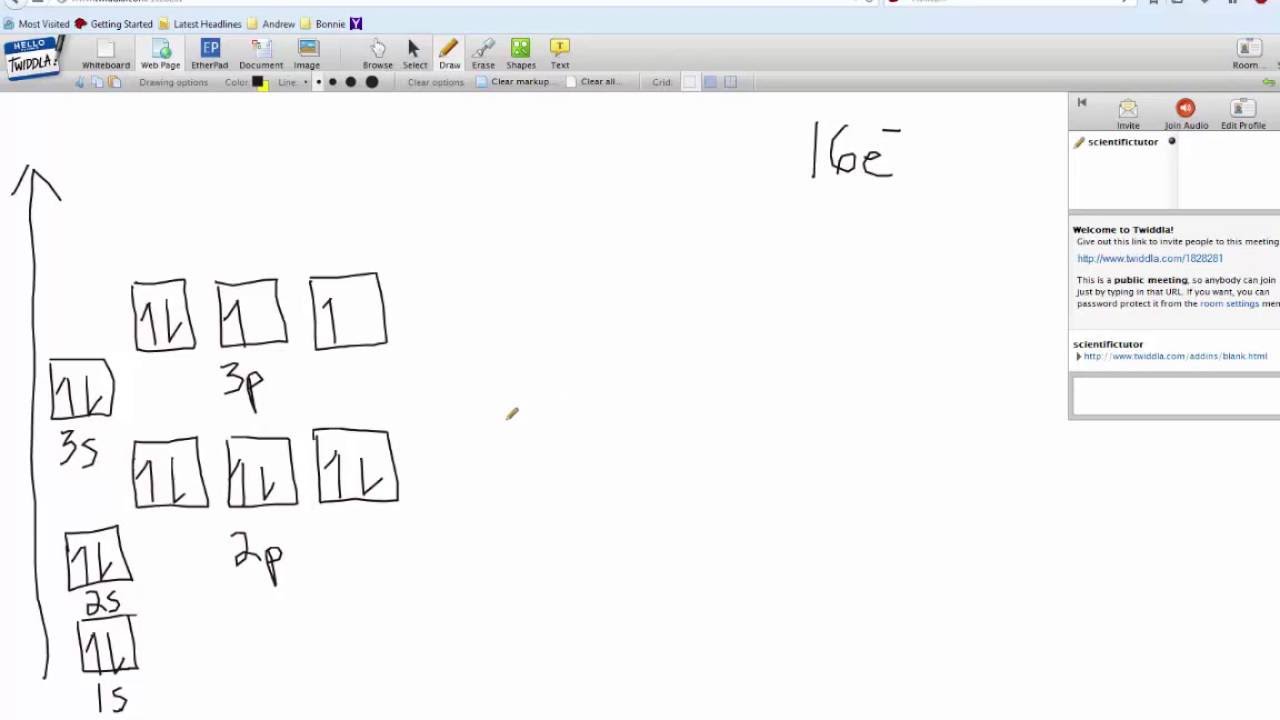
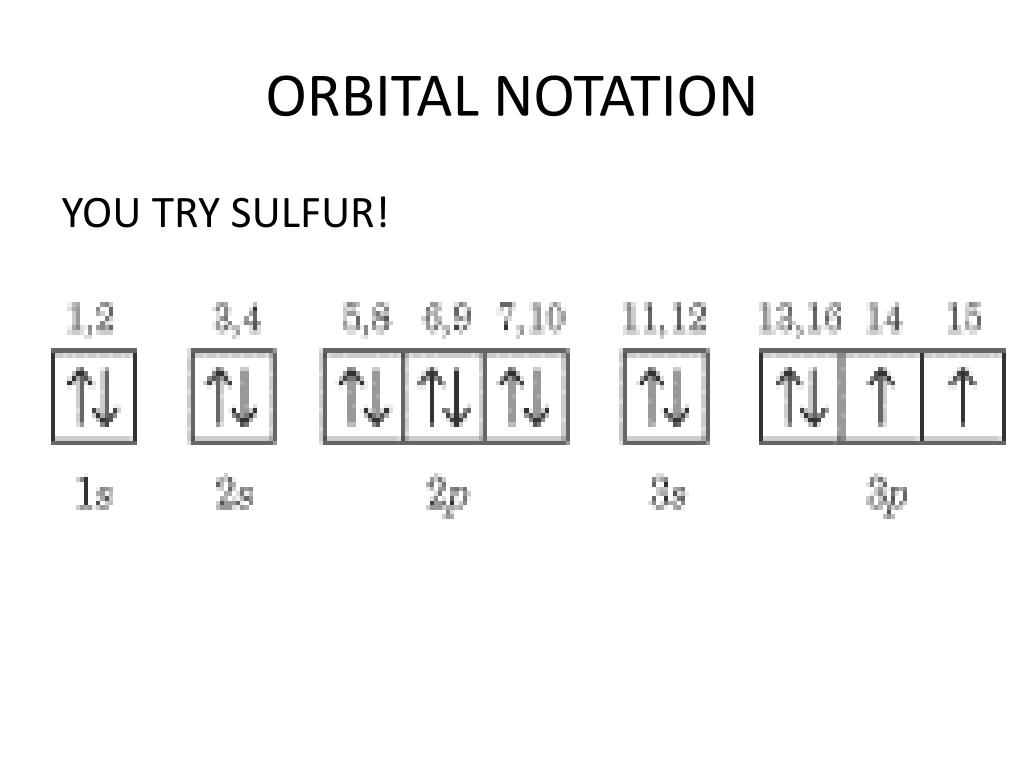
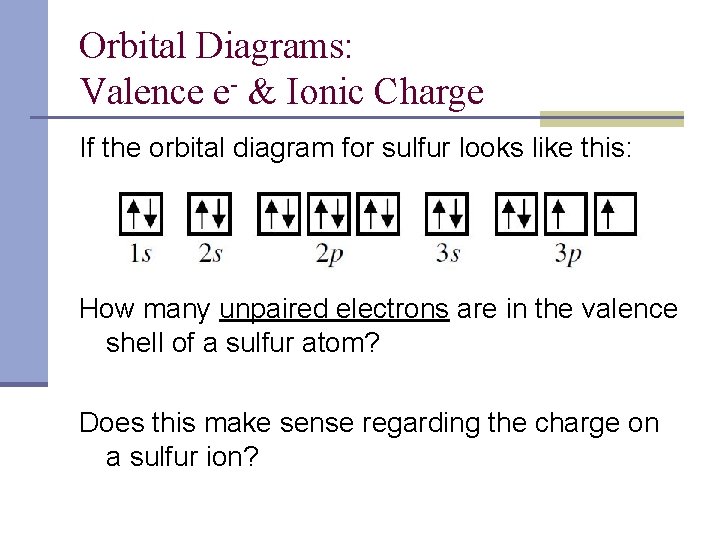
40 orbital diagram for sulfur
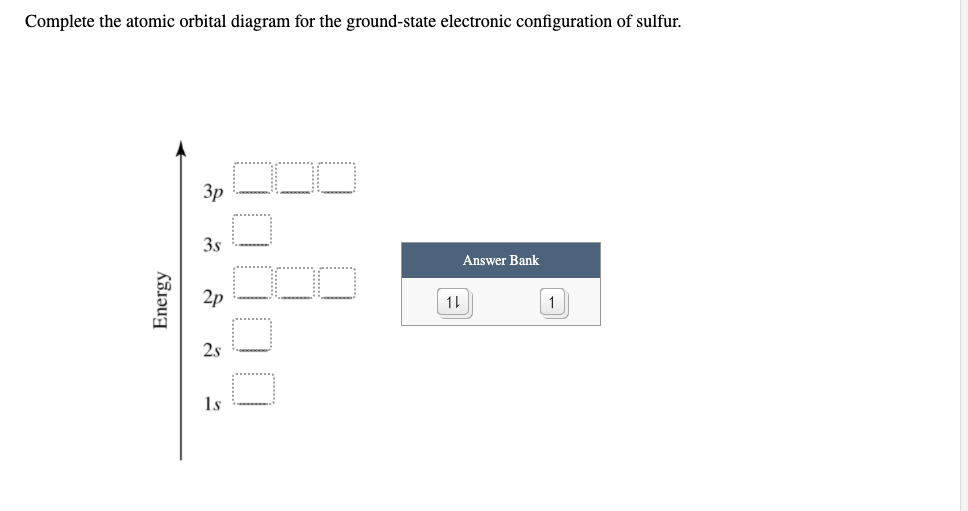
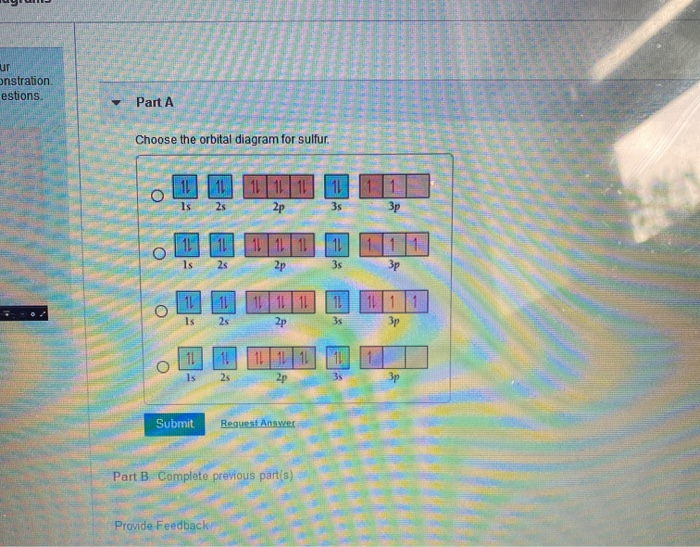
Solved Choose the orbital diagram for sulfur. 11 11 11 ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (1 rating) Transcribed image text: Choose the orbital diagram for sulfur. 11 11 11 111111 2p 11 3p ls 2s 3s 11 O 11 11 1s 2s 11 11 11 2p 11 3p 3s 1L 11 11 2s 11 11 11 2p 1 ls 3s 3p о 11 ls 11 2s 11 11 ... What is the orbital diagram for sulfur? - FindAnyAnswer.com In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. Click to see full answer Similarly, you may ask, what is the electron configuration of sulfur? [Ne] 3s² 3p4
Copper(Cu) electron configuration and orbital diagram Therefore, an electron of 4s orbital completes a full-filled 3d orbital by jumping into a 3d orbital. So, the copper(Cu) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. How to write the orbital diagram for copper(Cu)? To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion ...

Orbital diagram for sulfur
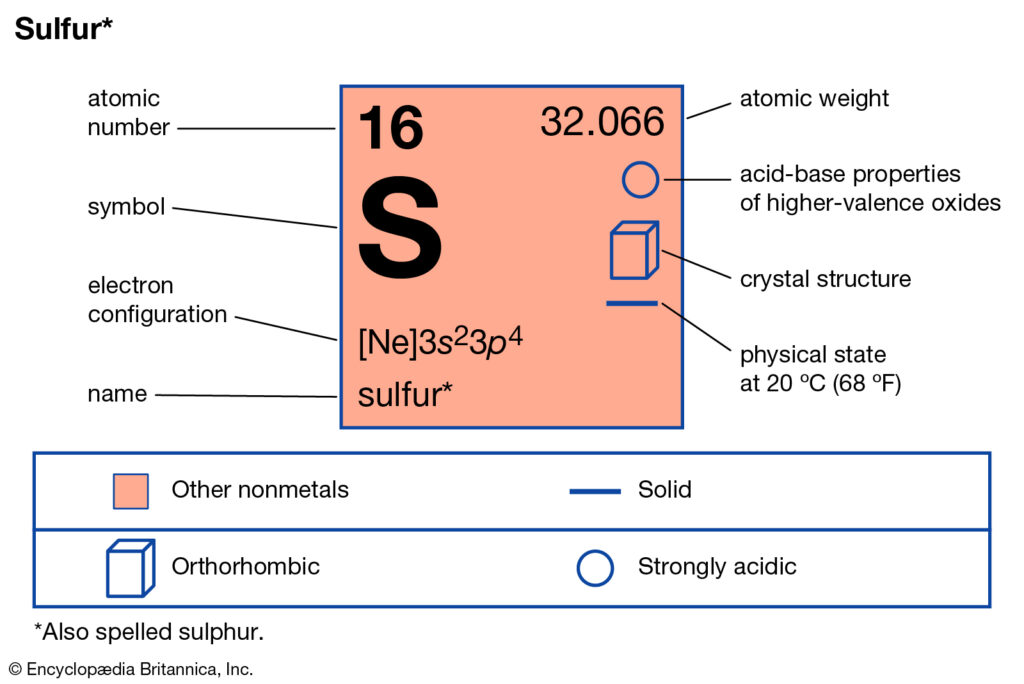
SO2 Lewis Structure, Hybridization, Molecular Geometry ... SO2 Molecular Orbital Diagram. The molecular orbital diagram of SO2 is attached below: A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound. In this MO we can see ... Electron configuration for Sulfur (element 16). Orbital ... Density: 2.06 g/cm 3 . Electronic configuration of the Sulfur atom: 1s 2 2s 2 2p 6 3s 2 3p 4. Reduced electronic configuration S: [Ne] 3s 2 3p 4. Below is the electronic diagram of the Sulfur atom Distribution of electrons over energy levels in the S atom. 1-st level (K): 2. 2-st level (L): 8. Oxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is 'O'. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this.
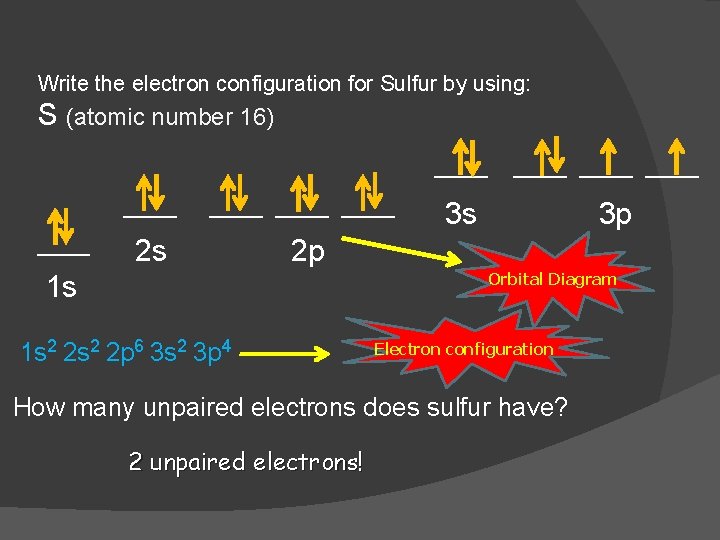
Orbital diagram for sulfur. Quick Answer: What Is The Orbital Diagram For Sulfur ... Orbital diagrams are a pictorial description of electrons in an atom. How many orbital levels does sulfur have? Sulfur has two electrons in the 1s orbital, two electrons in the 2s orbital, and six electrons in the 2p orbitals. What is the chemical symbol of Sulphur? S What is a ground state orbital diagram? SF4 Lewis Structure, Molecular Geometry, Hybridization ... SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 ... Hybridization of Atomic Orbitals - University of Illinois ... The fluorine atoms are sp 3 hybridized (3 lone pairs and one bonding pair), and the overlap of each sp 3 orbital on fluorine with a dsp 3 orbital on sulfur will form a s bond. For compounds, like SF 6, which require six equivalent molecular orbitals, mix six atomic orbitals, s + p + p + p + d + d. Electron Configuration for Sulfur (S) - UMD When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital.
Show The Orbital Filling Diagram For Sulfur - schematron.org Each arrow represents one electron.Aug 04, · The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Show The Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Solved Show the orbital-filling diagram for S (sulfur ... Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 1 15 2s 2p 3s 3p G1 G1 G1 G1G1 G1 G1 G1 | G1 G2 G2 G2 G2 G2 Submit Part D Show the orbital-filling diagram for Br (bromine). Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur ...
Electron Configuration Orbital Diagram Sulfur - YouTube To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use. What is the correct orbital diagram for sulfur? - Answers The correct orbital diagram of this element sulfur? We can't make diagrams on answers.com. Sulphur's configuration is 2, 8, 6. You may either use this information or refer to the element's page on ... diagramweb.net › orbital-filling-diagram-forOrbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. schematron.org › orbital-box-diagram-for-sulfurOrbital Box Diagram For Sulfur - schematron.org Mar 14, 2019 · on Orbital Box Diagram For Sulfur. Well, we use the aufbau principle, and for sulfur, Z= The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. boxes or lines represent each orbital. • arrows within boxes Draw an orbital diagram for beryllium (Z=4).
How to Write the Orbital Diagram for Sulfur (S) - YouTube To write the orbital diagram for the Sulfur atom (S) first we need to write the electron configuration for just S. To do that we need to find the number of e...
Orbital Box Diagram For Sulfur Orbital Box Diagram For Sulfur boxes or lines represent each orbital. • arrows within boxes Draw an orbital diagram for beryllium (Z=4). 1s. Guidelines for a) Sulfur (Z=16) b) Iron (Z=26). In order to write the Sulfur electron configuration we first need to know the When we write the configuration we'll put all 16 electrons in orbitals around the.
Answered: What is the orbital diagram for the… | bartleby What is the orbital diagram for the atom Sulfur? Question. What is the orbital diagram for the atom Sulfur? check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here. Want to see this answer and more? Experts are waiting 24/7 to provide step-by-step solutions in as fast as 30 minutes!*
topblogtenz.com › sulfur-orbital-diagram-electronSulfur Orbital diagram, Electron configuration, and Valence ... The Sulfur orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, and the remaining four electrons in 3p orbital. Orbital diagram for a ground-state electron configuration of a Sulfur atom is shown below- What is the electron configuration of the S2- ion?
Sulfur(S) electron configuration and orbital diagram Orbital diagram for sulfur (S) Sulfur (S) excited state electron configuration Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The valency of the element is determined by electron configuration in the excited state.
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
› science › orbital-diagram-sulfurWhat Is the Orbital Diagram for Sulfur? - Reference.com The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
SF2 Lewis Structure, Molecular Geometry, Hybridization ... SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of 70.062 g/mol, this compound is made up of one Sulfur atom and two Fluoride atoms. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or ...
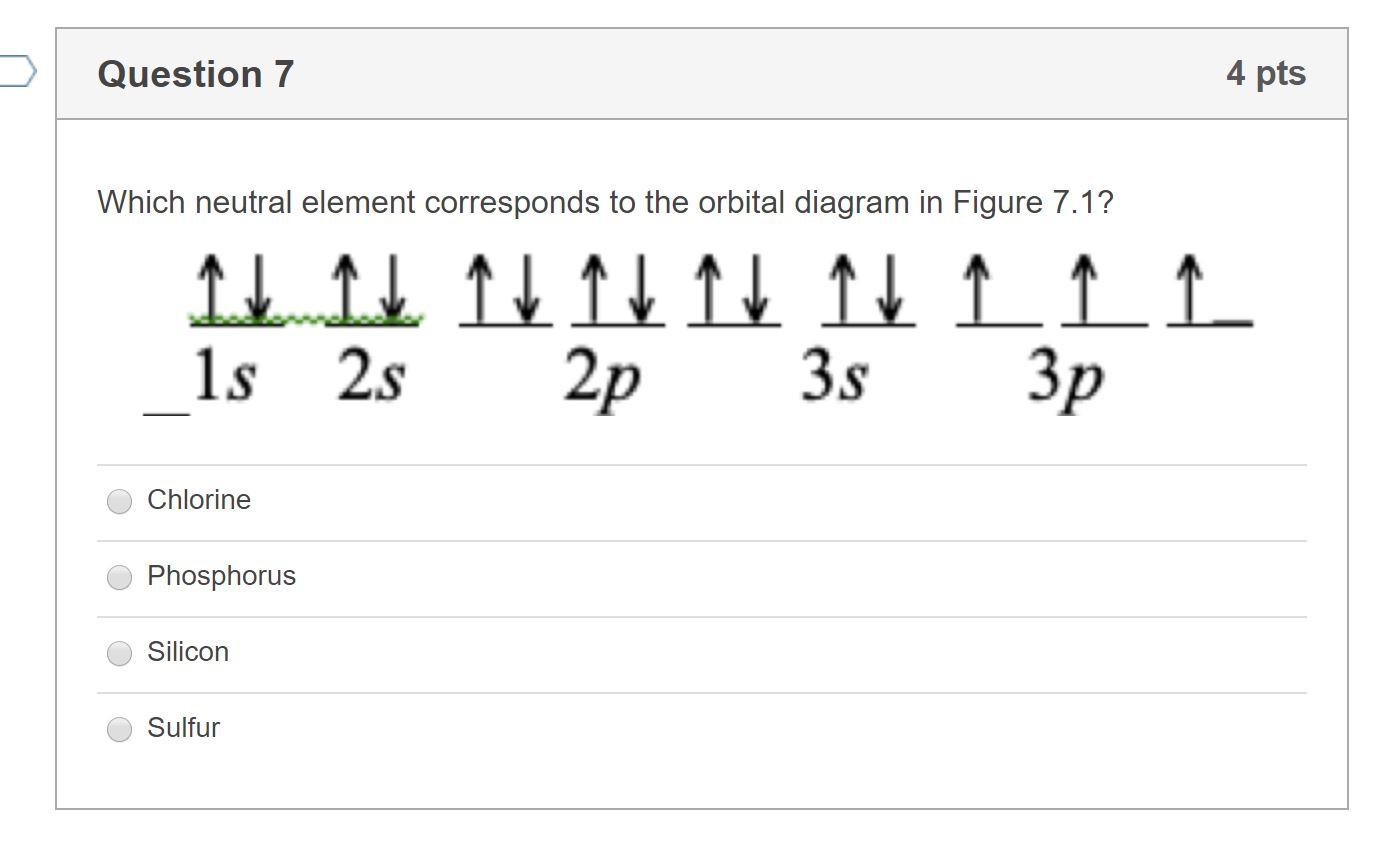
Orbital Diagrams Flashcards - Quizlet Gravity. Mg (Magnesium) Click card to see definition 👆. Tap card to see definition 👆. What element is represented by this orbital diagram? Click again to see term 👆. Tap again to see term 👆. S (Sulfur) Click card to see definition 👆.
Aluminum Orbital diagram, Electron configuration, and ... The orbitals are 1s, 2s, 2p, 3s, and 3p. The Aluminum orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, and the remaining one electron in 3p orbital. Orbital diagram for a ground-state electron configuration of an Aluminum atom is shown below-.
What is the orbital notation of Sulfur? - Answers What is sulfur's orbital notation? 1s2 + 2s2 + 2p6 + 3s2 + 3p4 = sulfur's orbital notation. What is the orbital notation for P? The orbital notation for P is 1s22s22p63s23p3.
Oxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is 'O'. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this.
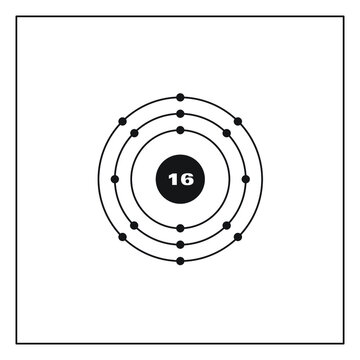
Electron configuration for Sulfur (element 16). Orbital ... Density: 2.06 g/cm 3 . Electronic configuration of the Sulfur atom: 1s 2 2s 2 2p 6 3s 2 3p 4. Reduced electronic configuration S: [Ne] 3s 2 3p 4. Below is the electronic diagram of the Sulfur atom Distribution of electrons over energy levels in the S atom. 1-st level (K): 2. 2-st level (L): 8.
SO2 Lewis Structure, Hybridization, Molecular Geometry ... SO2 Molecular Orbital Diagram. The molecular orbital diagram of SO2 is attached below: A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound. In this MO we can see ...









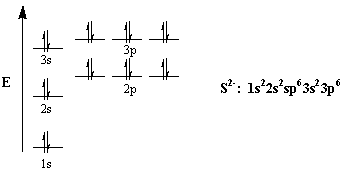











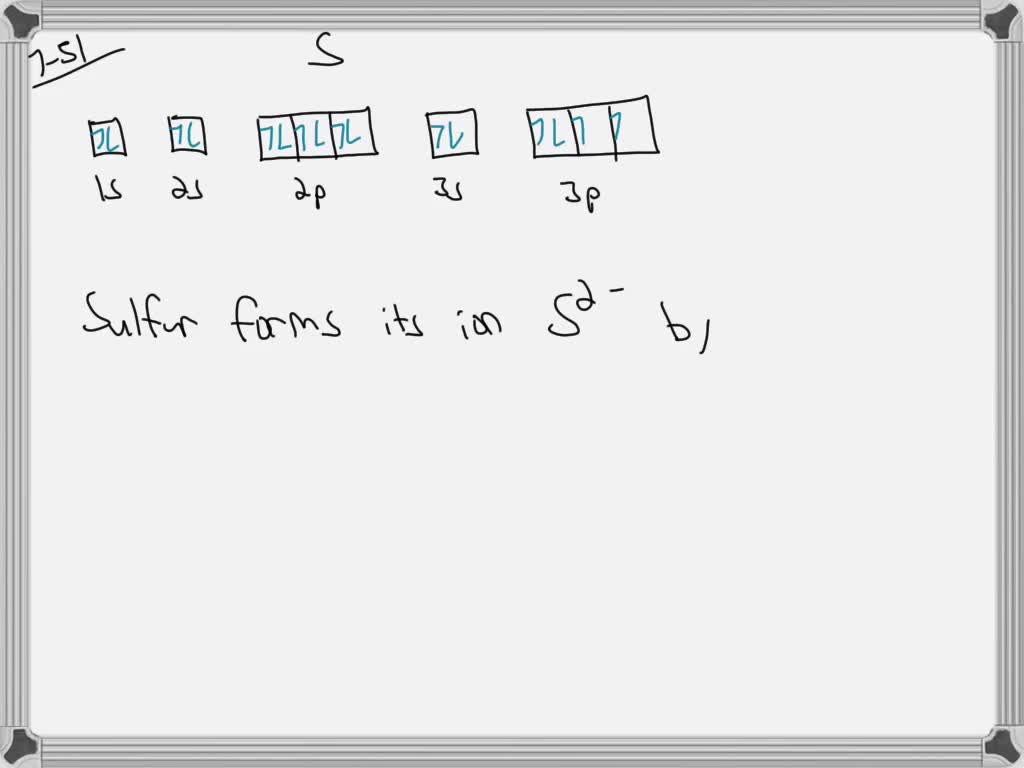

0 Response to "40 orbital diagram for sulfur"
Post a Comment