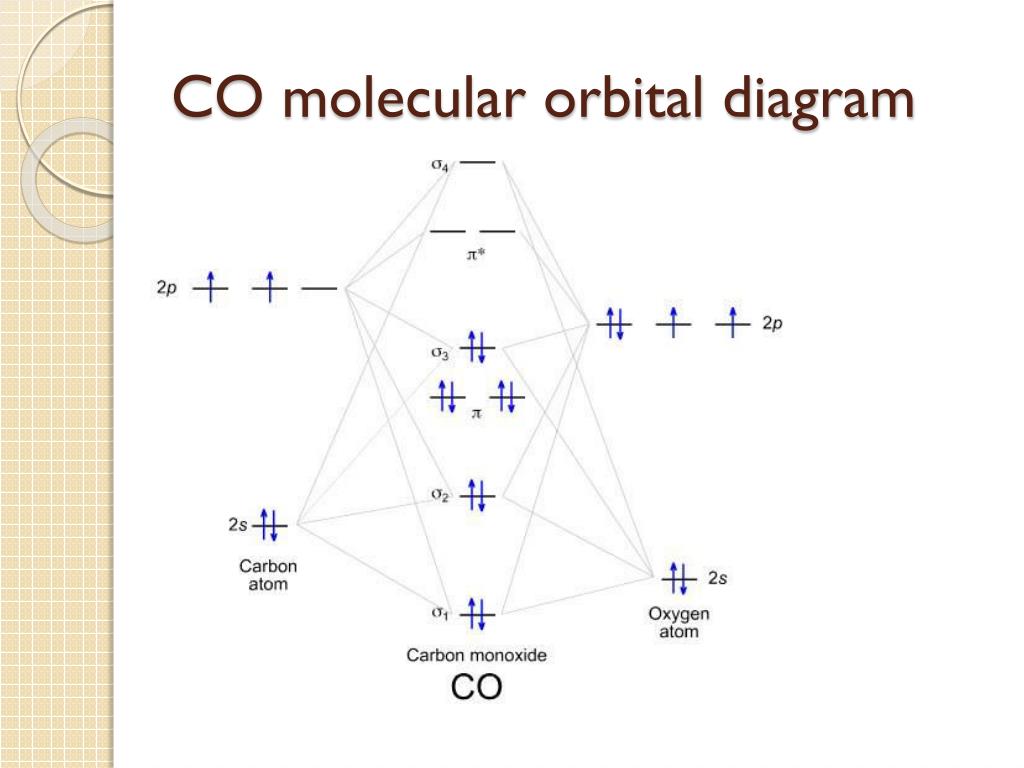
41 co molecular orbital diagram
CO2 Lewis Structure (2021 UPDATED) All You Need To Know Molecular Orbital (MO) Diagram In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2. Bond Angle Nh3 Mo Diagram - symmetry cisplatin c2v, nh3 tpd profile ... N2 2 Molecular Orbital Diagram. Molecular Orbital Of Co. NH3 Molecule. CH2 MO Diagram. Gallery of Nh3 Mo Diagram. Harford County Humane Society Saint Bernard Rescue Land Rover Portland Black Russian Terrier Round Bathroom Rugs Bunnies For Adoption Gingivitis In Cats Carol House Furniture Pick A Part Inventory Pendant Light Shade Inflatable Dog ...
Chemical Bonding MCQ And Answer With FREE PDF b) A molecular orbital is singly occupied. c) An example is oxygen molecule. d) Repelled by the magnetic field. Answer: Repelled by the magnetic field. 48. Combination of two atomic orbitals results in the formation of two molecular orbitals namely. a) one bonding and one non-bonding orbital. b) two bonding orbitals. c) two non-bonding orbitals
Co molecular orbital diagram
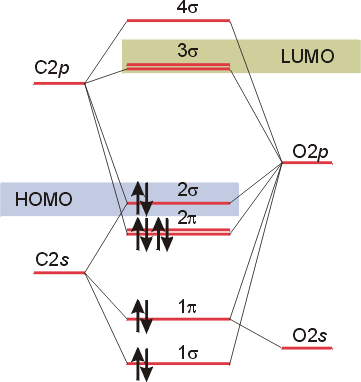
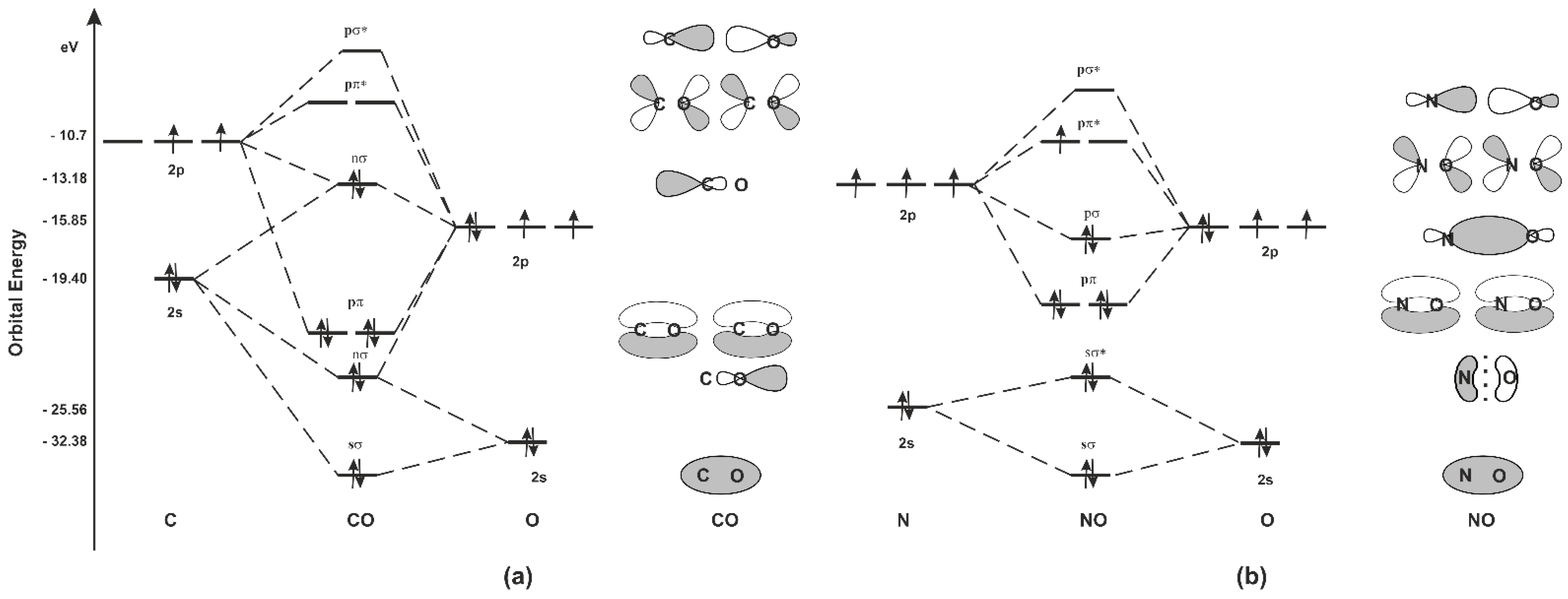
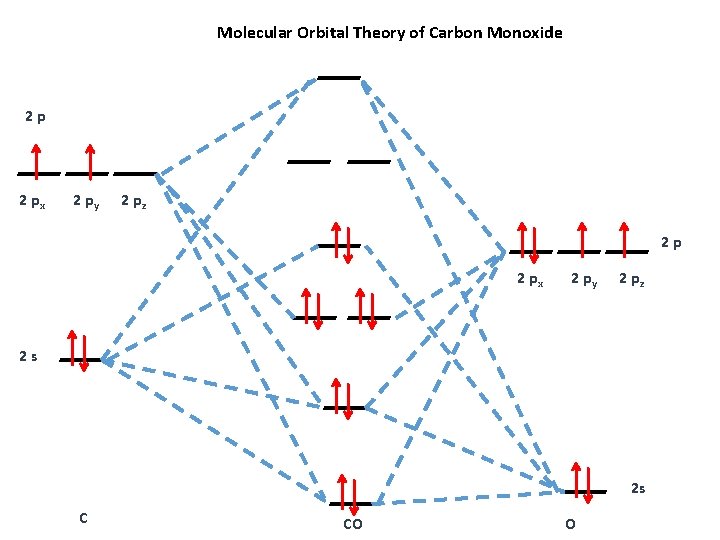
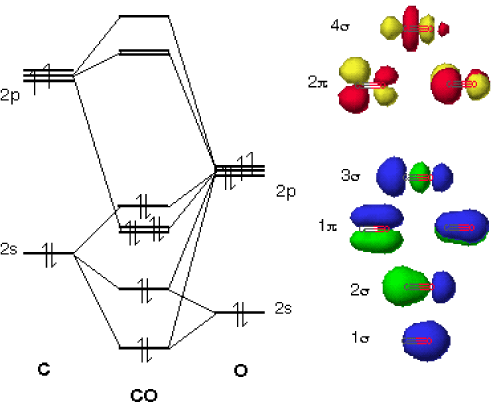
techiescientist.com › co-lewis-structureCO Lewis Structure, Geometry, and Hybridization - Techiescientist Feb 24, 2022 · Molecular Orbital Diagram of Carbon Monoxide (CO) The above image shows energy levels for the molecular orbitals of the carbon monoxide (CO) The molecular orbital diagram is a diagrammatic representation of showing how chemical bonding is taking place within a molecule. 42 complete this molecular orbital diagram for cn - Wiring ... Co molecular orbital diagram bond order. the Hale-Bopp comet. The valence-shell molecular or-bital diagram for SO is shown on the right. Complete the molecular orbital diagram by (a) identifying which atom is on the left side and which is on the right side, (b) labeling the valence-shell atomic orbitals with their appropriate ns and np ... Bond Order Formula: Definition, Calculation, Problems - Embibe The molecular orbital \({\rm{\sigma }}\) formed by the addition of atomic orbitals is called the bonding molecular orbital, and \({{\rm{\sigma }}^ * }\) by subtraction of atomic orbitals is called an antibonding molecular orbital. The antibonding orbital energy is raised above the parent atomic orbitals energy that has combined.
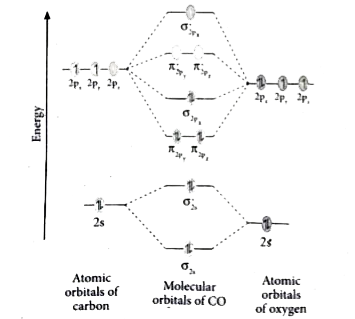
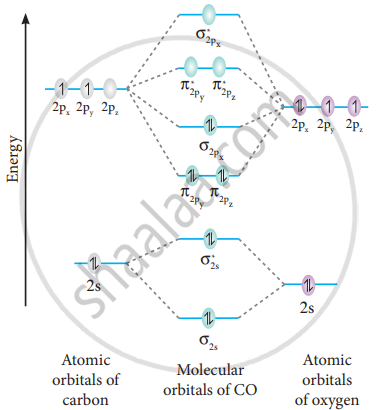
Co molecular orbital diagram. How many molecular orbitals are in o2 ... O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals. What Is the Molecular Orbital Theory? (with pictures) Phil Riddel Date: February 07, 2022 The Periodic Table of Elements.. Molecular orbital theory, or MO theory, is a method of explaining bonding between atoms in terms of electrons being spread out around a molecule rather than localized around the atoms, in contrast to valence bonding theory, or VB theory.Electrons in atoms are arranged in orbitals within subshells within shells. Three-dimensional tomographic imaging of CO molecular ... In order to retrieve the full orbital function, a 3D molecular orbital tomography (MOT) method is developed and is successfully applied to the reconstructions of the HOMO of CO, which simplifies the 3D imaging process of orbitals of linear molecules, and is expected to be extended to reconstruct the 3D orbitals of nonlinear molecules. Understanding the Role of Electronic Effects in CO on the ... CO adsorption on an ordered surface enables us to monitor the band dispersion change due to the interaction between CO molecular orbitals and surface electronic structures of Pt-Sn alloys. In comparison with DFT calculations, the enhanced spectral features near the E F are identified as d xz and d yz orbitals of the π bonding.
What covalent compound is co? - Colors-NewYork.com A polar covalent bond between carbon and oxygen is a carbon-oxygen bond. Oxygen has 6 valence electrons and chooses either to share two carbon bonding electrons, leaving the 4 non-bonding electrons in 2 lone pairs: O: or to share two electron pairs to form the functional group of carbonyl. Understanding the Role of Electronic Effects in CO on the ... CO adsorption on an ordered surface enables us to monitor the band dispersion change due to the interaction between CO molecular orbitals and surface electronic structures of Pt−Sn alloys. In comparison with DFT calculations, the enhanced spectral features near the E F are identified as d xz and d yz orbitals of the π bonding. 39 complete an orbital diagram for boron. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. 31.12.2021 · This is the detailed Periodic table with electron configuration where you can find the electron configuration of all the elements written in the table itself. how many electrons does a co atom have in its 3d subshell ... CO Molecular Geometry Carbon monoxide is a linear molecular geometry, there is a triple bond between C and O, and each atom contains one lone pair of electrons. Carbon and oxygen form one sigma bond and two pi bonds. ... how many neutrons does co have fill in the atomic orbital diagram for nitrogen. how many subshells are in the n=2 shell?
Chemical Bonding and Molecular Structure Class 11 ... In an anti-bonding molecular orbital, electron density is minimum. (a) around one atom of the molecule. (b) between the two nuclei of the molecule. (c) at the region away from the nuclei of the molecule. (d) at no place. Answer. B. Question. When two atomic orbitals combine, they form. Molecular Orbital Theory: Tutorial and Diagrams - Video ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B_2^2+, B2, C_2^2-, B_2^2- and N_2^2+ b. Draw the Lewis structures and molecular orbital diagrams for (Get Answer) - Construct the molecular orbital diagram for ... Cation Anion Formula Name Magnesium bicarbonate SrCl2 Selection A Manganese II) chlorate 2+ Co PO4 3- Selection B Cu2CO3 Selection C Construct the molecular orbital diagram for N2 and then identify the bond order Bond order 0.5 O 1.5 O 2.5 2s 2s... Valence Bond Theory in Coordination Compounds - GeeksforGeeks Examples of Octahedral complexes. Inner Orbital Complexes: [Co(CN) 6] 3-ion The oxidation state of cobalt in this combination is +3. The electrical configuration of the Co +3 valence shell is 3d 6.; Because the CN - ligands are strong, they cause the pairing of 3d-electrons.; As a result, all six 3d electrons are coupled and occupy three of the five 3d orbitals.
9.7: Molecular Orbitals - Chemistry LibreTexts Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
en.wikipedia.org › wiki › MoleculeMolecule - Wikipedia A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
www2.chemistry.msu.edu › faculty › reuschMolecular Structure & Bonding - Michigan State University In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond.
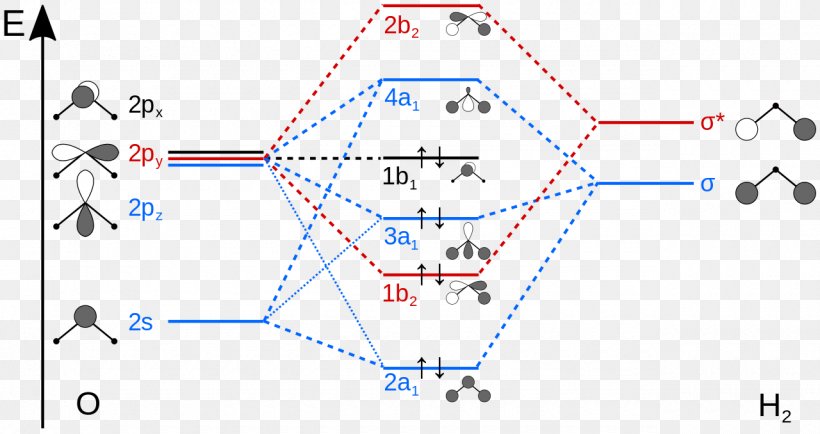
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Co molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).according to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰ Label orbitals sigma, sigma*, pi or pi*.
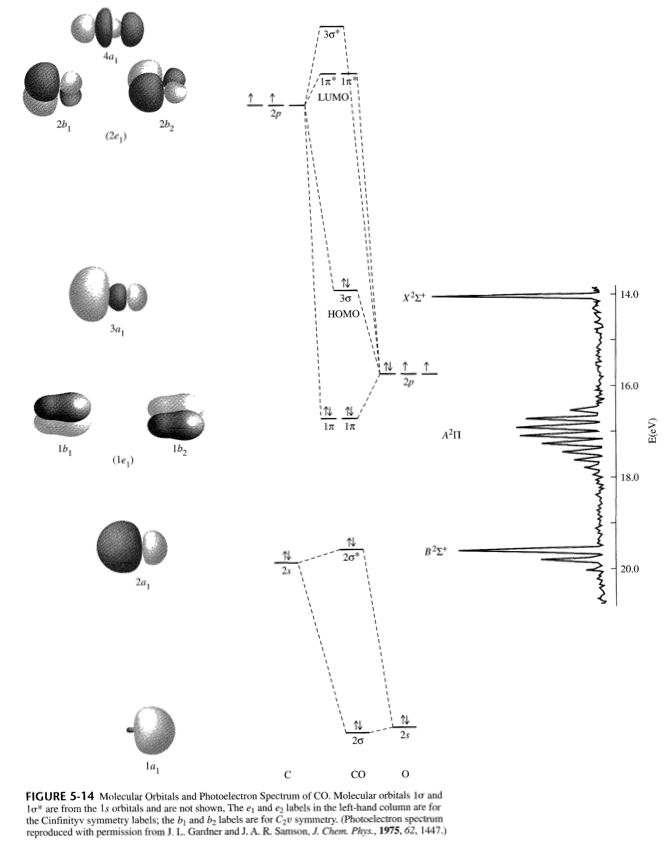
Consider the heteronuclear diatomic molecule CO. The ... Consider the heteronuclear diatomic molecule CO. The ionization energies of an electron from the valence atomic orbitals on the carbon atom and the oxygen atom are listed below. Use these data to construct a molecular orbital energy-level diagram for CO. What are the symmetry designations of the...
Molecular orbitals diagrams of [Co(NH3)6]3+ Molecular orbitals diagrams of [Co(NH3)6]3+ 1. M. O. diagram for [Co(NH3)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Co3+→[Ar] 3d6, 4s0 6e- Ligand group (NH3) orbitals 6 x 2 = 12 e- σ [Co(NH3)6]3 ...
› ~lawm › Ch 5 SolutionsMiessler-Fischer-Tarr5e SM Ch 05 CM diagram for CO2 in Figure 5.25 can be used as a guide, with the orbitals of Be higher in energy than those of C and the orbitals of F lower in energy than those of O. Calculated molecular orbital shapes are below, for comparison for those of CO 2 in Figure 5.25.
Molecular Orbital Theory Concept & Diagrams | What is ... Molecular orbitals are the result of adding the different atomic orbitals together constructively and destructively. Each atomic orbital can be assigned a negative or positive value, or sign; so ...
Bonding in Metal Carbonyls: Explaination, Type, Property ... The molecular orbital configuration of the CO molecule is : (σ1sb)2(σ1 s ∗)2(σ2sb)2(π2Pxb = π2Pyb)4(σ2Pzb)2(σ2s ∗)2(π2Px ∗ = π2Py ∗)0(σ2Pz ∗)0 Learn 12th CBSE Exam Concepts In the molecular orbital of CO 1. The highest occupied molecular orbital (HOMO) that can donate the lone pair of electrons to form an OC → Mσ bond is σ2 s ∗. 2.
Sigma vs Pi bond: The Identifications and Main Differences ... The number of molecular orbitals produced must always be equal to the number of atomic orbitals combined. The interaction between atomic orbitals, for example, 1s orbitals of the hydrogen molecule forming bonding and antibonding molecular orbitals can be represented as the energy level diagram. After the construction of the energy level diagram ...
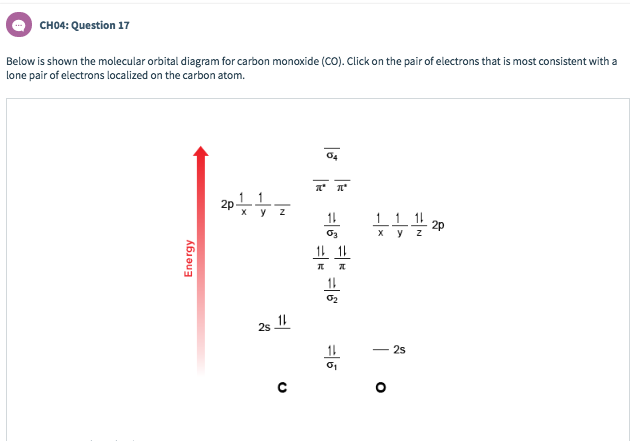
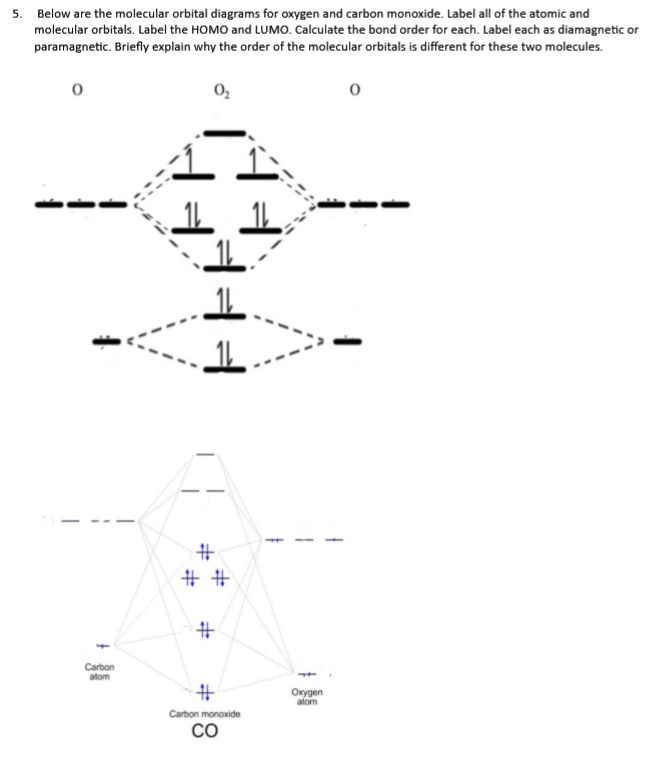
socratic.org › questions › by-writing-molecularBy writing molecular orbital configuration for NO,CO,O2 ... Mar 18, 2018 · "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram.
pressbooks-dev.oer.hawaii.edu › phase-diagramsPhase Diagrams – Chemistry - University of Hawaiʻi Consider the phase diagram for carbon dioxide shown in as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating ...
en.wikipedia.org › wiki › Molecular_cloudMolecular cloud - Wikipedia The evidence comes from the fact that the "turbulent" velocities inferred from CO linewidth scale in the same manner as the orbital velocity (a virial relation). Physics [ edit ] The Serpens South star cluster is embedded in a filamentary molecular cloud, seen as a dark ribbon passing vertically through the cluster.
Molecular Orbital Theory - Chemical Bonding and Molecular ... Molecular orbital theory Features of Molecular orbital theory 1) The atomic orbitals overlap to form new orbitals called molecular orbitals. When two atomic orbitals overlap or combine ,they lose their identity and form new orbitals. The new orbitals thus formed are called molecular orbitals. 2) Molecular orbitals are the energy states of a molecule in […]
Molecular orbitals diagram for ML6 - slideshare.net 1. Molecular orbital Diagram for ML6 Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. nd Six donor ligand orbitals (n-1)s (n-1)p Δo Metal atomic orbitals [ML6] molecular orbitals M. O. diagram for [ML6] complex (no π interactions) Antibonding MOs Bonding MOs Non bonding MOs.
inorganic chemistry - Effect of metal identity on CO bond ... In organometallic carbonyl complexes, a back-donation effect occurs: the σ molecular orbital of CO yields electron density to an orbital of the appropriate metal atom, and in turn, a d orbital of appropriate symmetry yields electron density to the antibonding π* orbitals of CO.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36:
What Is The Bond Order Of B2 1So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond.Nov 11, 2016
Bond Order Formula: Definition, Calculation, Problems - Embibe The molecular orbital \({\rm{\sigma }}\) formed by the addition of atomic orbitals is called the bonding molecular orbital, and \({{\rm{\sigma }}^ * }\) by subtraction of atomic orbitals is called an antibonding molecular orbital. The antibonding orbital energy is raised above the parent atomic orbitals energy that has combined.
42 complete this molecular orbital diagram for cn - Wiring ... Co molecular orbital diagram bond order. the Hale-Bopp comet. The valence-shell molecular or-bital diagram for SO is shown on the right. Complete the molecular orbital diagram by (a) identifying which atom is on the left side and which is on the right side, (b) labeling the valence-shell atomic orbitals with their appropriate ns and np ...
techiescientist.com › co-lewis-structureCO Lewis Structure, Geometry, and Hybridization - Techiescientist Feb 24, 2022 · Molecular Orbital Diagram of Carbon Monoxide (CO) The above image shows energy levels for the molecular orbitals of the carbon monoxide (CO) The molecular orbital diagram is a diagrammatic representation of showing how chemical bonding is taking place within a molecule.













0 Response to "41 co molecular orbital diagram"
Post a Comment