41 li2+ molecular orbital diagram
Solved Construct the molecular orbital diagram for Li2 ... Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. Li Li Li Answer Bank 2s 2s、、 02s Identify the bond order O 0 O 05 O 1 O 1S 02.5. Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Use the molecular orbital diagram shown to determine which ... Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.

Li2+ molecular orbital diagram
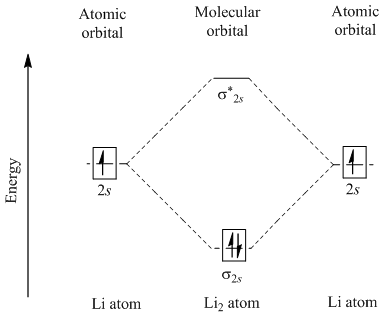
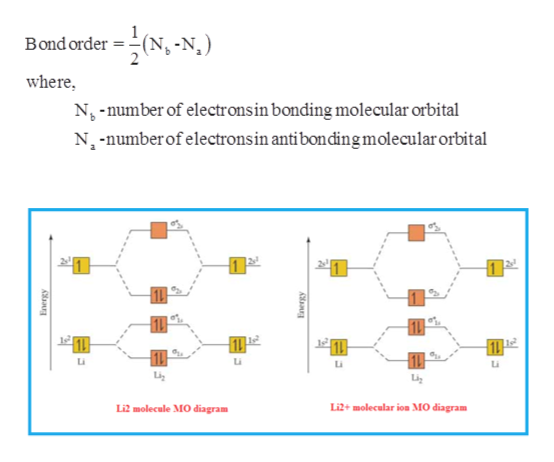
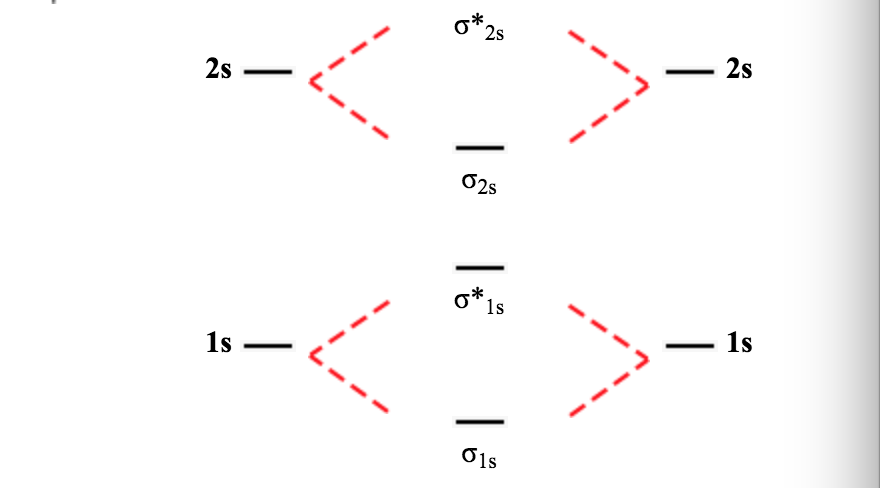
7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ... Solved Molecular orbitals 1. Below is presented the ... Solved Molecular orbitals 1. Below is presented the | Chegg.com. Molecular orbitals 1. Below is presented the molecular orbital diagram for Li2 a) What is the bond order for this molecule? b) Which of the molecular orbitals will have nodal planes? What does this imply about these orbitals (the ones with.
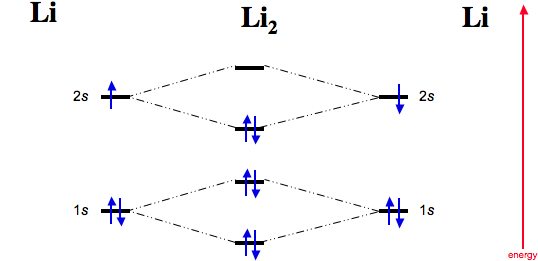
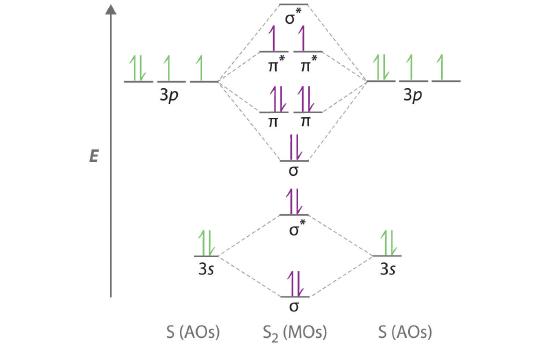
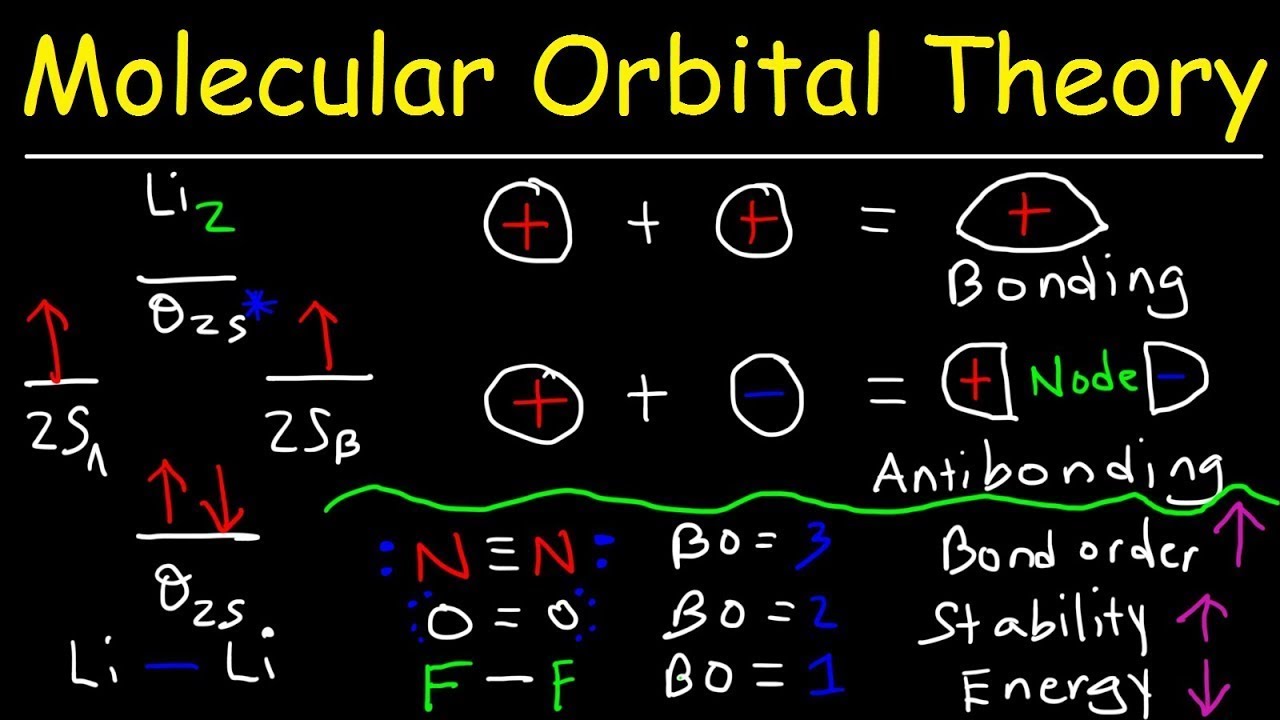
Li2+ molecular orbital diagram. Li2- Molecular Orbital Diagram Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. How to determine gerade & ungerade symmetry of a MO ... J.D.Lee writes in his book Concise Inorganic Chemistry:. An alternative method for determining the symmetry of the molecular orbital is to rotate the orbital about the line joining the two nuclei and then rotate the orbital about the line perpendicular to this.If the sign of the lobes remains the same, the orbital is gerade, and if the sign changes, the orbital is ungerade. Li2- Molecular Orbital Diagram - schematron.org The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule . Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. Molecular Orbital Diagram Of N2 N2 Ions And Li2 Molecule ... the pi(2p) bonding orbitals are lower than the sigma(2p) bonding orbitals. n2(2 ) has a bonding order of 2, which predicts that Related image with molecular orbital diagram of n2 n2 ions and li2 molecule
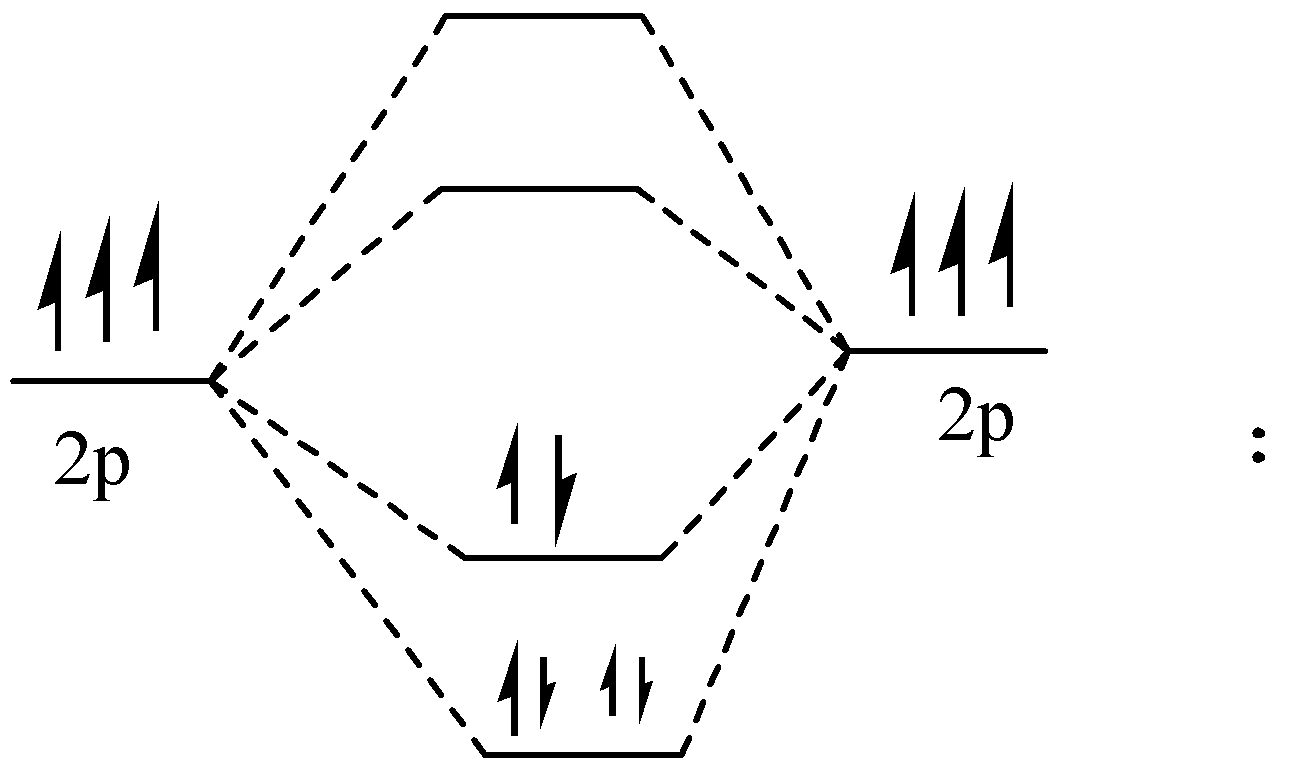
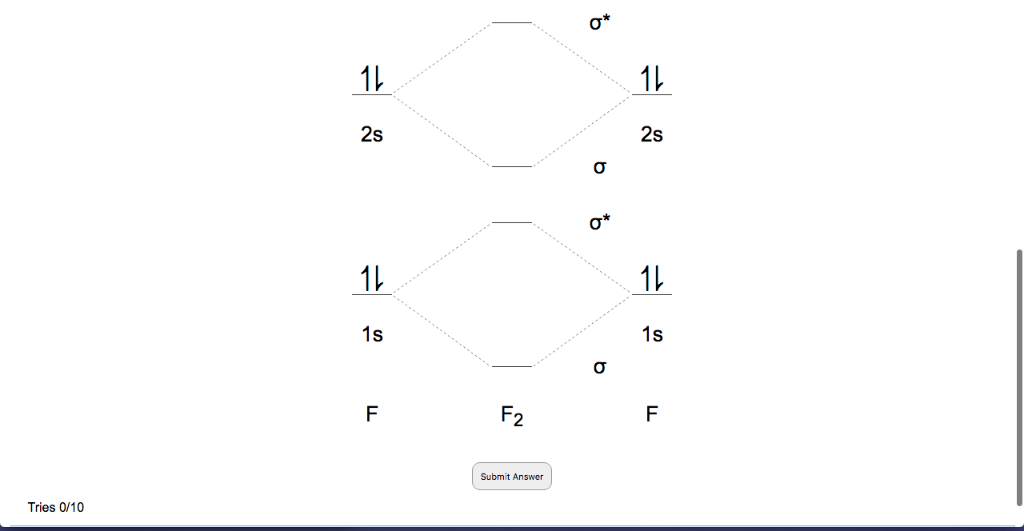
Bonding in Homonuclear Diatomic Molecules: Li2, Li2+ ... Get access to the latest Bonding in Homonuclear Diatomic Molecules: Li2, Li2+, Be2, B2, C2, N2 prepared with IIT JEE course curated by undefined on Unacademy to prepare for the toughest competitive exam. PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the Which of the following is paramagnetic? (use the mole... Which of the following is paramagnetic? (use the molecular orbital diagram) a) Li 2. b) Be 2. c) B 2. d) C 2. e) N 2. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos. Molecular Orbitals - Introductory Chemistry - 1st Canadian ... The head-to-head overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10").
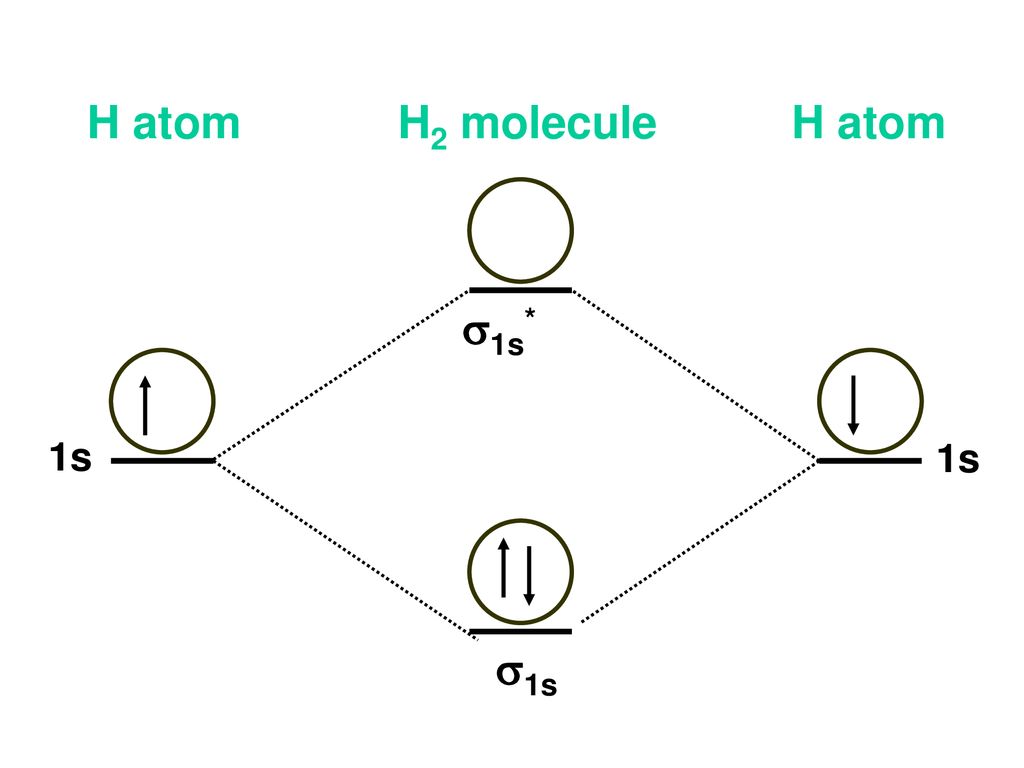
Molecular orbital : A molecule in which all the electrons ... Molecular orbital. source : chemed.chem.purdue.edu. Relationship between Electronic configuration and Molecular behaviour : 1) Bond order : It is defined as the number of covalent bonds between the two combining atoms of a molecule. What is the bonding order of Li2? - Quora In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combining two 1s orbitals,two molecular orbitals are formed among which ... How to predict the existence of Li2 C2 with their ... Answer: This is a very ambitious calculation. F. A. Matsen did such a calculation on the molecule Li-H about fifty years ago, but the calculations have become more streamlined since then. The results from such calculations will not actually predict the "existence" of Li2C2, but will provide a re... Li2 Molecular Orbital Diagram Molecular orbital energy level of Li2.This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as .
40 molecular orbital diagram for he2+ - Diagram For You The antibonding orbital is empty. Thus, H2 is a stable molecule. Construct the molecular orbital diagram for he2 Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each.
Is li2 - paramagnetic or diamagnetic? - TreeHozz.com A molecular structure that results in unpaired electrons in degenerate MOs, like O2, is called paramagnetic. Li2 has all of its electrons neatly paired up in the orbitals. Li2 is diamagnetic. All this is further explained here.
Why "Li"_2^+ is more stable than "Li"_2 ... - Socratic.org Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and
Molecular Orbital Diagram (MO Diagram) of Li2 - YouTube Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.Check me out:
Write the electronic configuration of Lithium (Li2 ... Reason The number of electrons in antibonding molecular orbitals is two less than in bonding molecule orbitals.
Molecular Orbital Diagram For Li2 - Wiring Diagrams Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
MOT | Molecular Orbital Energy level Diagram for Li2, Li2 ... This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o...
Molecular orbital theory - W3schools Molecular orbital theory : As long as the specific molecular orbitals forms (their dependency on R and Z in the cylindrical coordination system) vary for each and every molecule, their dependency on an angle 'f' due to the represented by quantum number L and their behavior of G or U w.r.t to inversion are entirely determined by system's geometry.
43 complete the atomic orbital (ao) and molecular orbital ... 43 complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.
Molecular Orbital Diagram For Li2 - schematron.org Oct 17, 2018 · Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure “Molecular orbital. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
Solved Molecular orbitals 1. Below is presented the ... Solved Molecular orbitals 1. Below is presented the | Chegg.com. Molecular orbitals 1. Below is presented the molecular orbital diagram for Li2 a) What is the bond order for this molecule? b) Which of the molecular orbitals will have nodal planes? What does this imply about these orbitals (the ones with.
Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.

















![Solved] Using Figures 9.35 and 9.43 as guides, draw the ...](https://s3.amazonaws.com/si.question.images/image/images11/876-(557)-2.png)


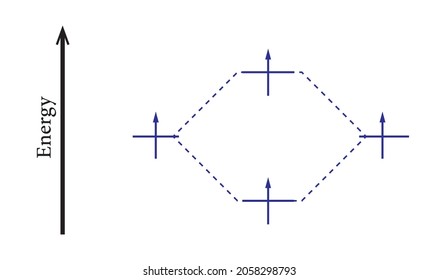
0 Response to "41 li2+ molecular orbital diagram"
Post a Comment