42 ethylene molecular orbital diagram
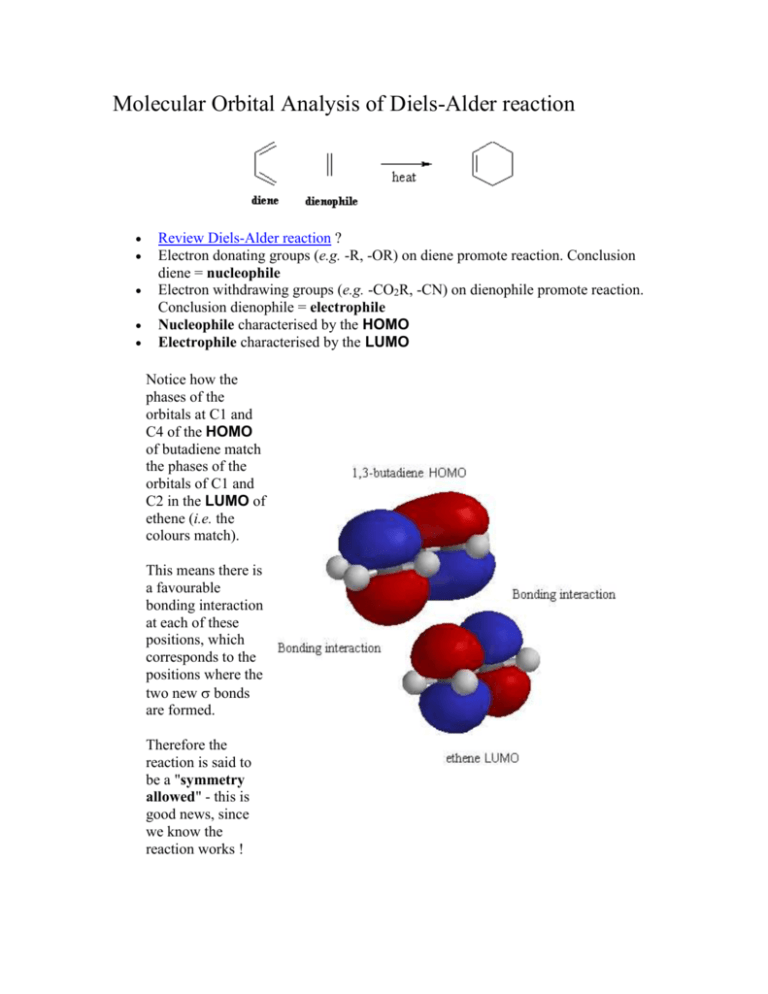
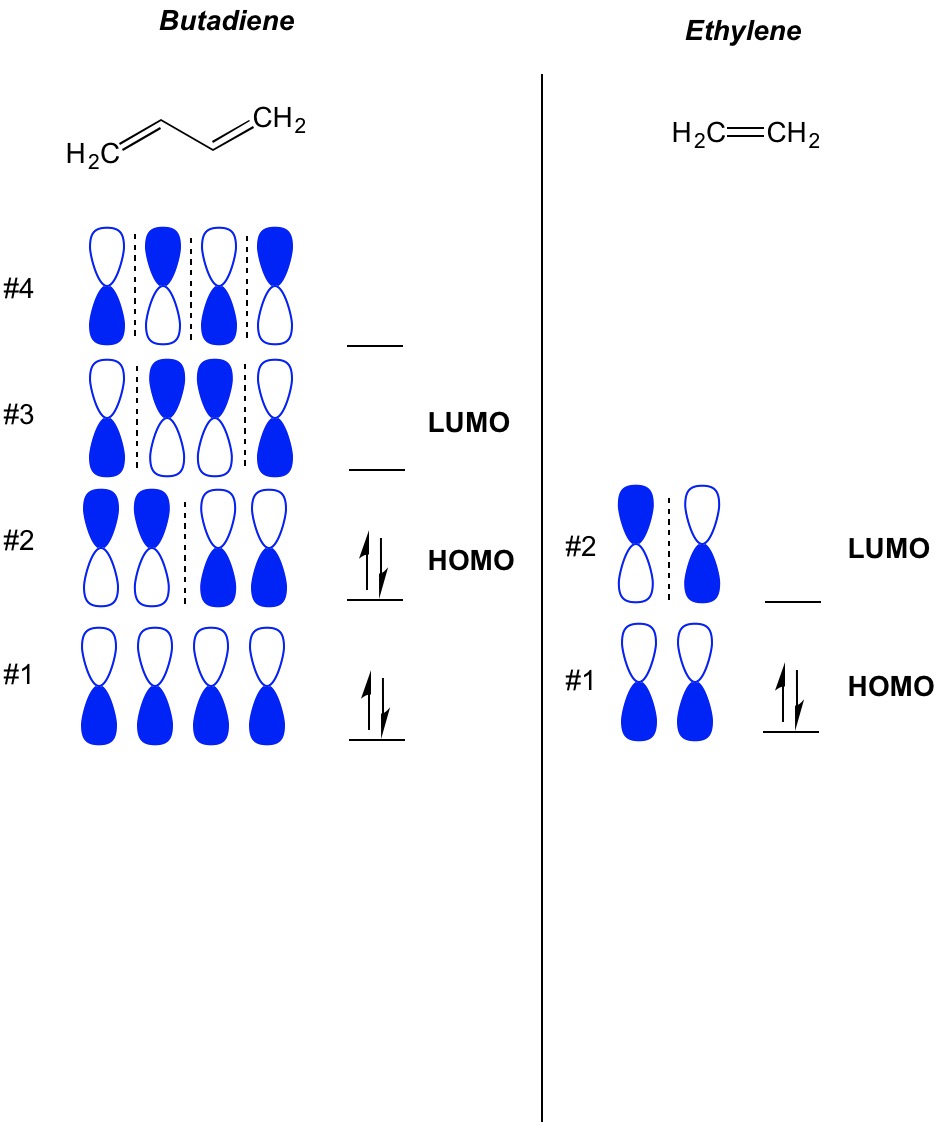
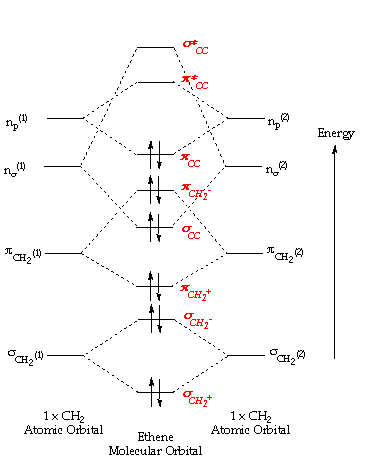
Molecular orbital energy level diagram for the two ... Molecular orbital energy level diagram for the two-ethylene system, before the molecules have come into contact, plotted with respect to the level degeneracy. The HOMO level has been set to the ... Molecular Orbital Diagram Of Ethene A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons.
16.2a Introduction to Pi Molecular Orbitals Ethylene - YouTube Chad introduces Pi Molecular Orbitals using Ethylene, drawing Bonding and Antibonding Molecular Orbitals and identifying the HOMO and LUMO.I've created an or...
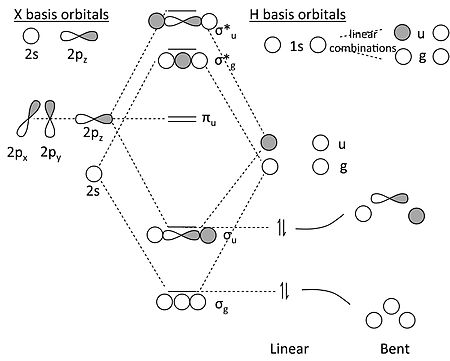
Ethylene molecular orbital diagram
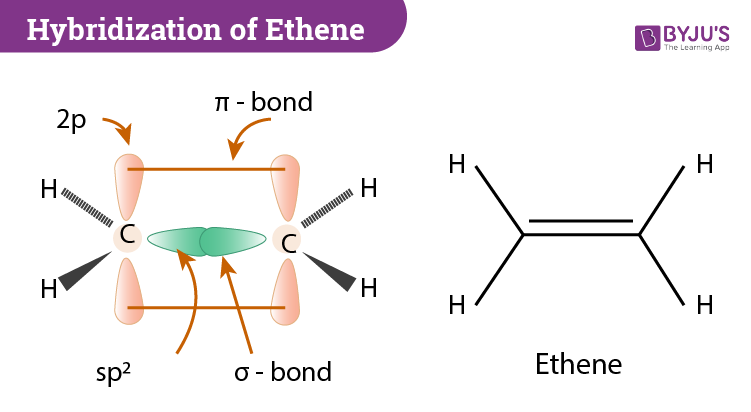
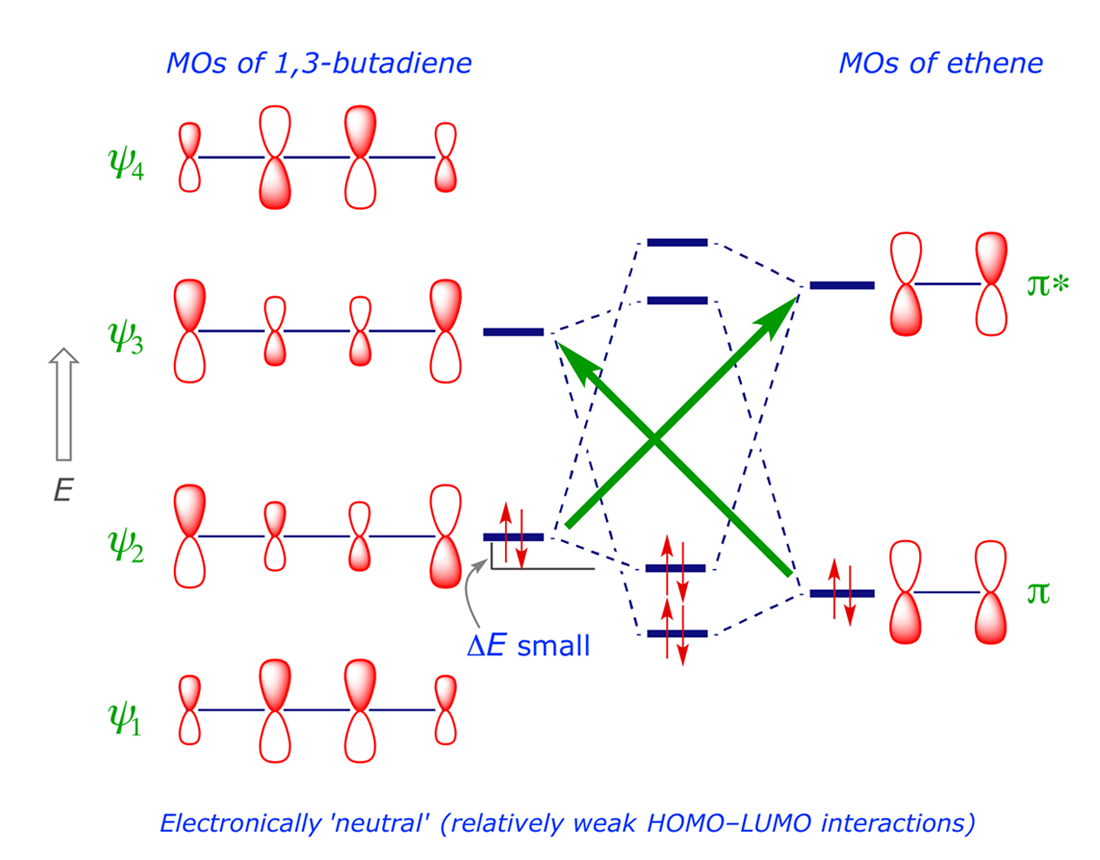
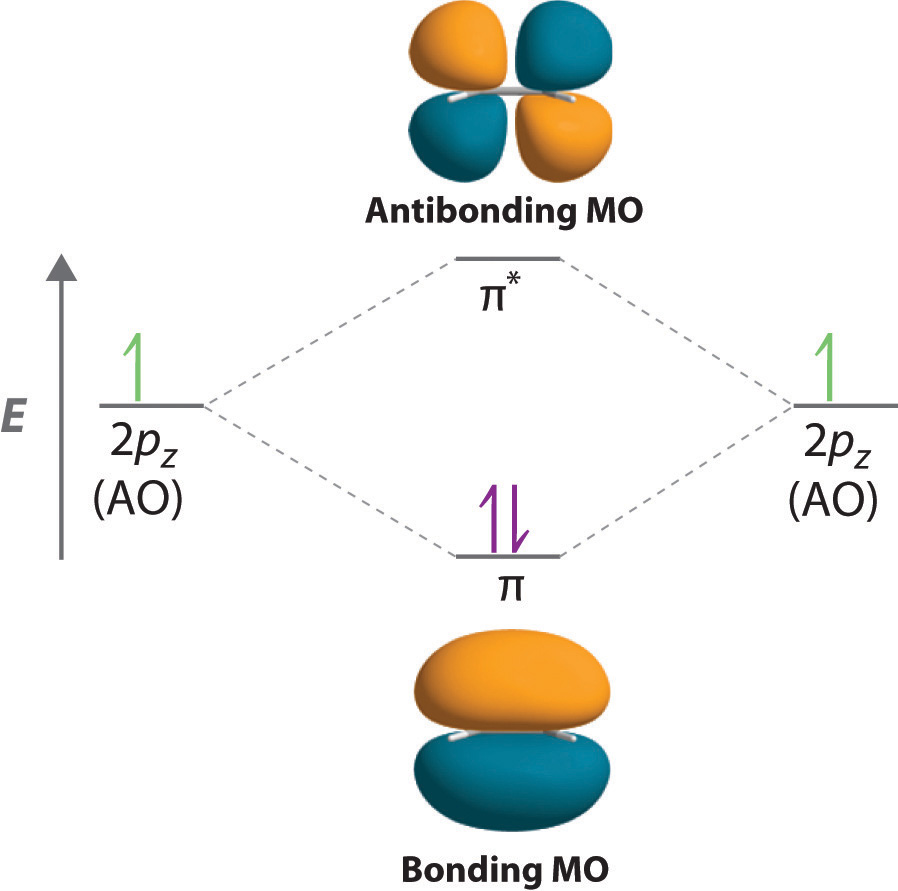
Pi Molecular Orbitals 1,3,5 Hexatriene - Chad's Prep® Pi Molecular Orbitals of 1,3,5-Hexatriene With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure. Introduction to Pi Molecular Orbitals Ethylene - Chad's Prep® Pi Molecular Orbitals of Ethylene In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals: ψ1 and ψ2*, (also referred to as π1 and π2*). Hybridization of C2H4 - Ethene (Ethylene) - BYJUS In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles. C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o.
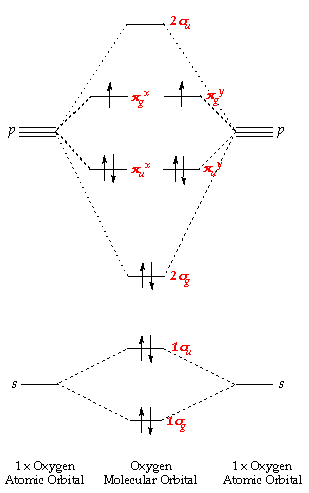
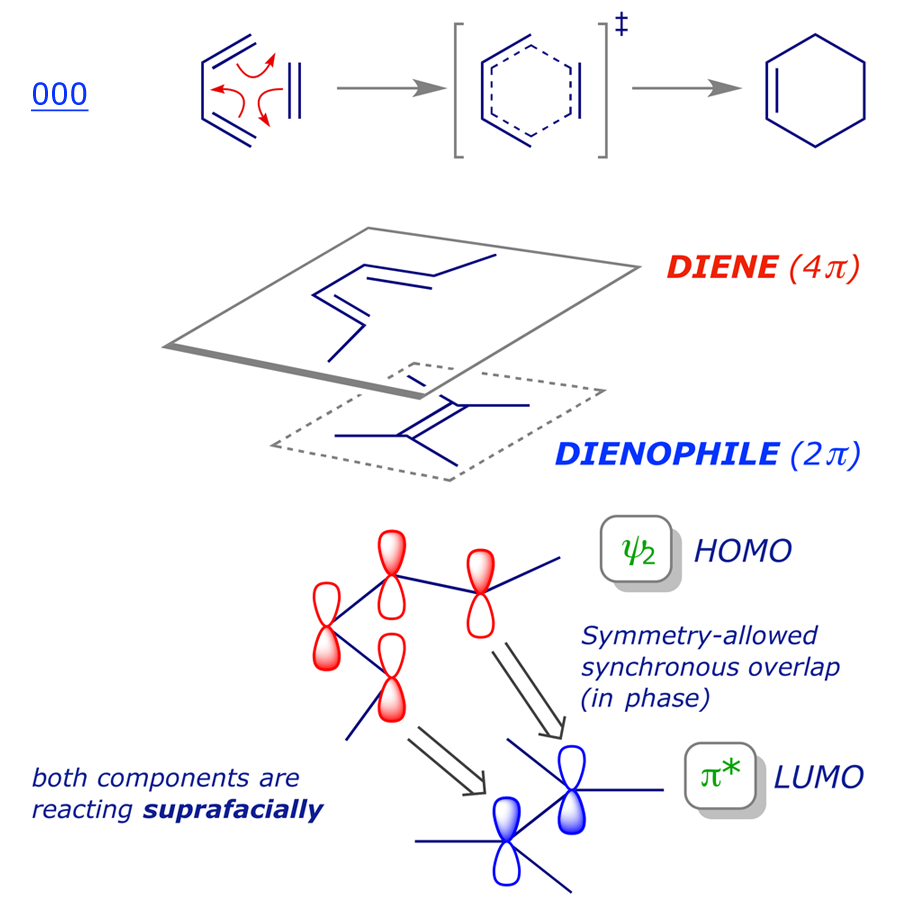
Ethylene molecular orbital diagram. Molecular Orbital theory: Ethane Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups. Ethyne Molecular Orbital Diagram - Wiring Diagrams An explanation of the bonding in ethyne (acetylene), including a simple view of hybridisation. In the diagram each line represents one pair of shared electrons. which are pointing towards each other now merge to give molecular orbitals.COMPOSITION OF ETHYNE MOLECULE: Ethyne molecule consists of two C-atoms and two H-atoms (C 2 H 2). › topics › chemistrySN2 Mechanism - an overview | ScienceDirect Topics Reaction Coordinate Diagram of an S N 2 Reaction. The point of maximum energy in the reaction coordinate diagram in Figure 9.3 is the transition state. It is the point of maximum energy on the pathway of minimum energy on the landscape from reactants to products. Molecular orbital diagrams of methylene | Download ... Molecular orbital diagrams of methylene. Source publication +5. Photogenerated reactive intermediates from thiophene ylides: thiophenes, oxenes and carbenes, oh my. Article. Full-text available.
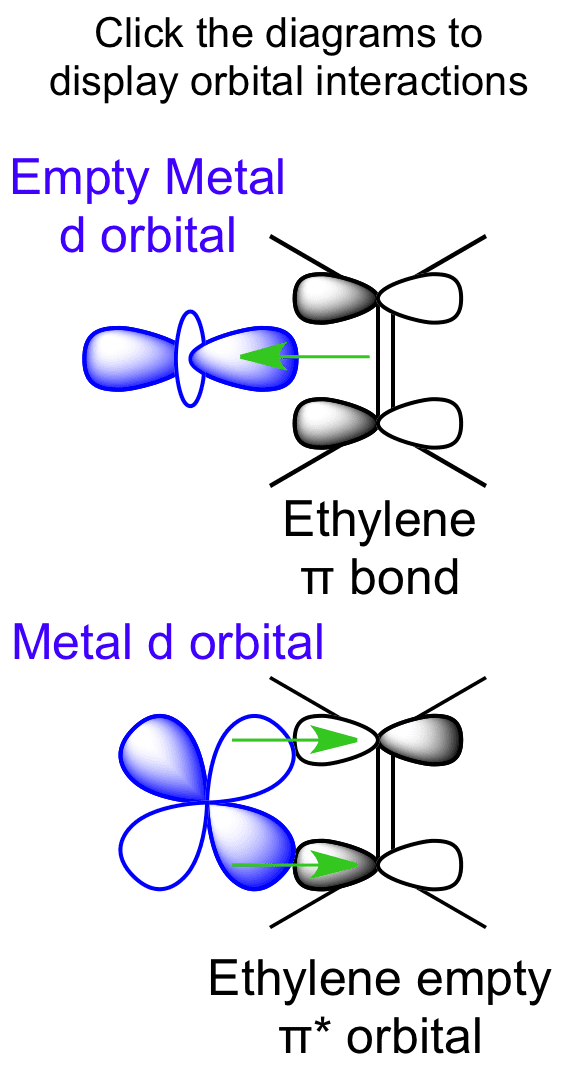
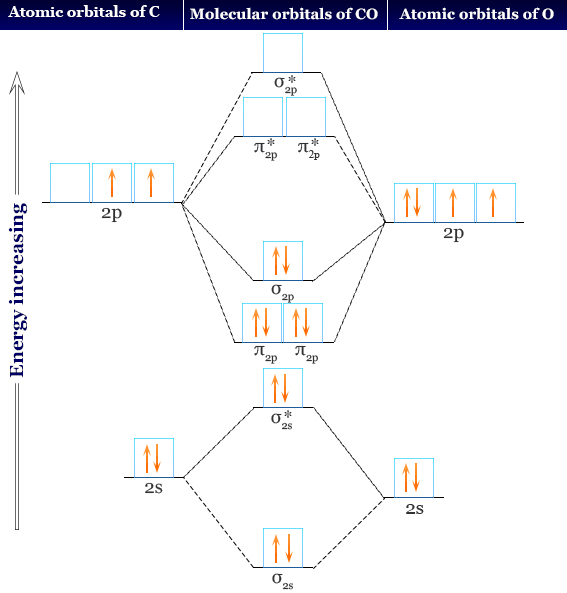
Molecular Orbital Diagram Of Ethene Ethene: The simplest alkene is ethene. Its chemistry is dominated by two "frontier orbitals", that is the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO. Ethene Molecular Orbital Diagram - schematron.org Ethene is built from hydrogen atoms (1s 1) and carbon atoms (1s 2 2s 2 2p x 1 2p y 1). A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. Interactions between Ethylene Molecular Orbitals and Metal ... Ethylene is capable of acting as a ligand as the C=C π bond can donate electron density to an empty metal d orbital, forming a σ bond. A filled metal d orbital is capable of donating electron density into the C=C π antibonding orbital. View Ethylene Molecular Orbitals here. Explore Metal-Ligand bonding with other molecules C2H4 Lewis Structure, Molecular Geometry, Hybridization ... The other sp2 hybrid orbitals form sigma bonds between C and H, therefore, leading to C-H single bonding structure. C2H4 Molecular Orbital (MO) Diagram. The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles.
Ethene Molecular Orbital Diagram - Wiring Diagrams A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons. Hybridization of C2H4 - Ethene (Ethylene) - BYJUS In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles. C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o. Introduction to Pi Molecular Orbitals Ethylene - Chad's Prep® Pi Molecular Orbitals of Ethylene In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals: ψ1 and ψ2*, (also referred to as π1 and π2*). Pi Molecular Orbitals 1,3,5 Hexatriene - Chad's Prep® Pi Molecular Orbitals of 1,3,5-Hexatriene With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure.


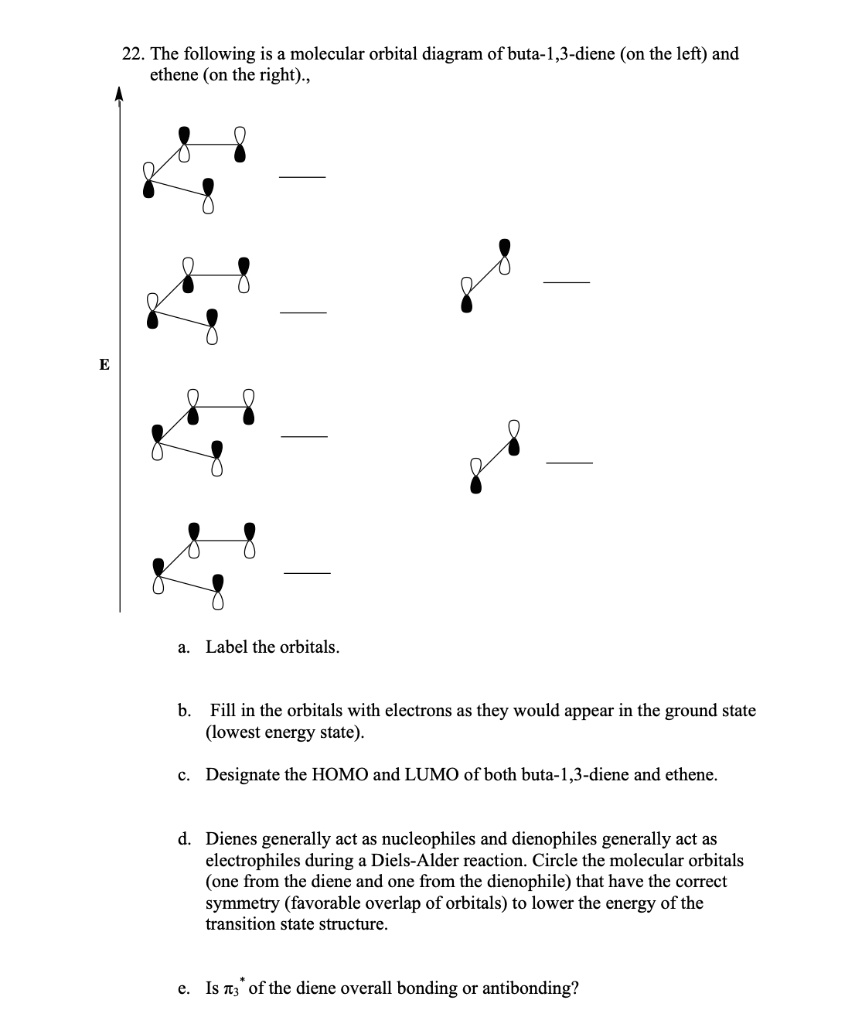

![Chuẩn] C2H4 Lewis Structure, Molecular Geometry ...](https://techiescientist.com/wp-content/uploads/2021/03/C2H4-MO-Diagram.jpg)











0 Response to "42 ethylene molecular orbital diagram"
Post a Comment