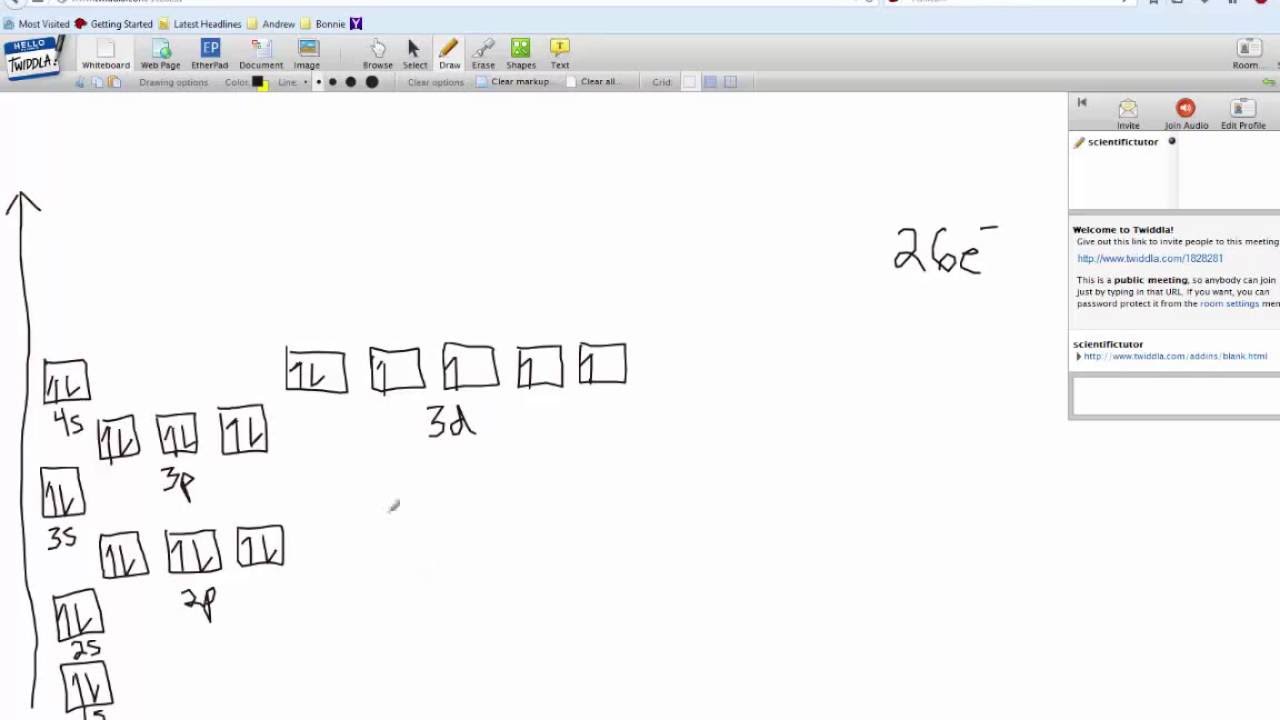
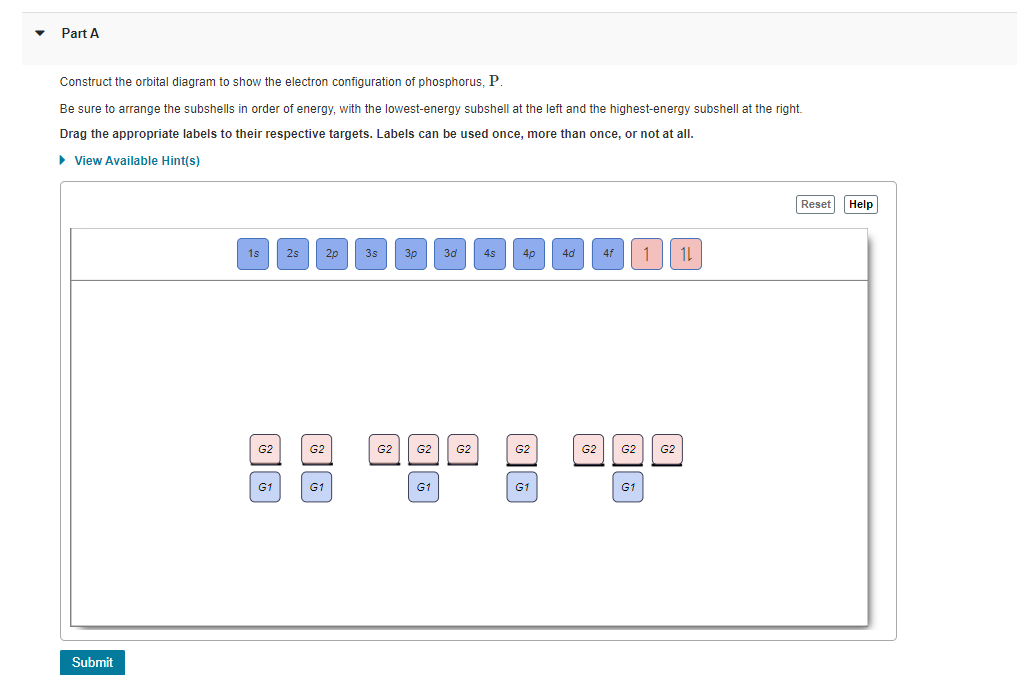
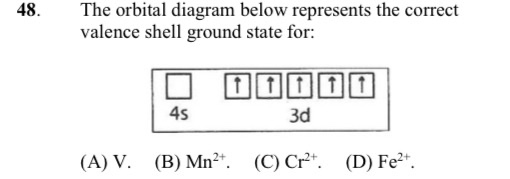
42 fe2+ orbital diagram
Electron Configuration Of Fe2+ - ViralListClub.com Au3 Xe 4f14 5d7 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. The outermost shell in this case is the 4s orbital. Sketch an atomic orbital diagram for Fe2 in its ground state. A normal Fe atom has an electron arrangement of. Paramagnetic With One Unpaired Electronb. The Ground State Electron Configuration Of Fe2 Ion Is 1s2 2s2 2p6 3s2 3p6 3d6 Therefore Fe2 Is. Molecular orbital (SCF-X-α-SW) theory of Fe 2+- Mn 3+ , Fe ... In this work, spin-unrestricted molecular orbital calculations on (FeMnO10) clusters are used to study the nature of magnetic exchange and electron delocalization (charge transfer) associated with Fe3+-Mn2+, Fe3+-Mn3+, and Fe2+-Mn3+ interactions in oxides and silicates. Molecular orbital (SCF-X-α-SW) theory of Fe 2+- Mn 3+, Fe 3+- Mn 2+, and ...
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Fe2+ orbital diagram
(Color online) The upper part shows the MO diagram of an ... Download scientific diagram | (Color online) The upper part shows the MO diagram of an Fe1-O-Fe2 complex inside GFO. The lower part of the figure sketches the corresponding DOS. Marked in red and ... 40 orbital diagram for fe - Diagram For You fe2+ orbital diagram - wiring diagrams a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) molecular orbital method in particular.electron configuration for fe2+ - chemistry … Orbital Diagram For Fe3+ - schematron.org Orbital Diagram For Fe3+. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). Fe3+ loses an electron from the d You take electrons out of the 4s orbital, before you take them out of the 3d orbital, because it is lower. -3,-2,-1,0,1,2,3 c.
Fe2+ orbital diagram. Fe2+ Orbital Diagram - Wiring Diagrams A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.electron configuration for Fe2+ - CHEMISTRY COMMUNITYMolecular orbital diagram - Wikipedia Draw molecular orbital diagram for F2 molecule Also class ... Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ... Electron Configuration for Iron (Fe, Fe2+, and Fe3+) After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ... Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
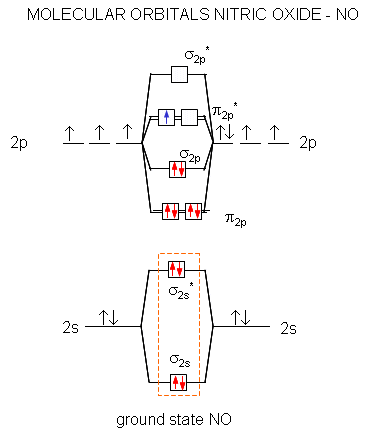
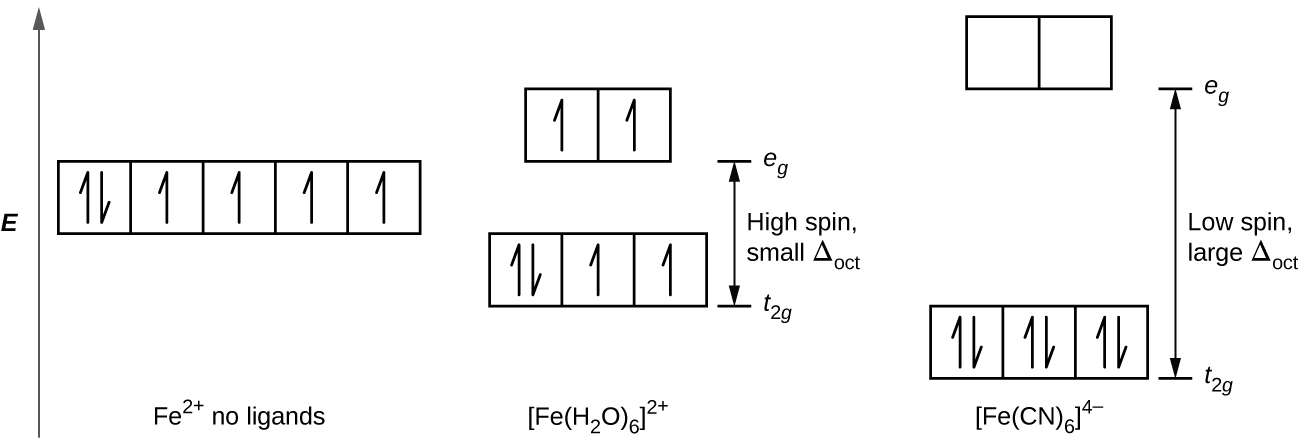
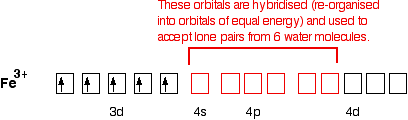
Molecular Orbital Diagram For Ne2 We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. What is the hybridisation of [Fe(H2O) 6] 2+? - Quora Answer (1 of 5): Find out the oxidation number of Fe. Hence, x (be the oxidation number of Fe) = +2 As H2O is neutral. Write down Fe2+ electronic configuration [Ar] 4s2 3d6 → Fe Hence, [Ar] 4s0 3d6 = Fe2+ (Acc. to Aufbou's Rule) As we know, H2O is a weak field ligand, no extra pairing will ... Pyrite oxidation and reduction: Molecular orbital theory ... The Fe2* t^v) orbitals and the S2" v* orbitals are filled. Electron transfer can only occur from a v* orbital (HOMO) ofSj-, the bridging ligand, to a partially filled ti^ie) orbital (LUMO) ofFe3"^ from the aquo complex (Fig. 2). The Fe3* reduces to Fe24- (d6, tige} ion). Half equations quiz questions - Footprints-Science | GCSE ... The lungs Quiz States of matter Quiz Chromatography Quiz GCSE Biology sample animations and quizzes GCSE Chemistry sample animations and quizzes GCSE Physics sample animations and quizzes GCSE Investigative Skills animations/slides
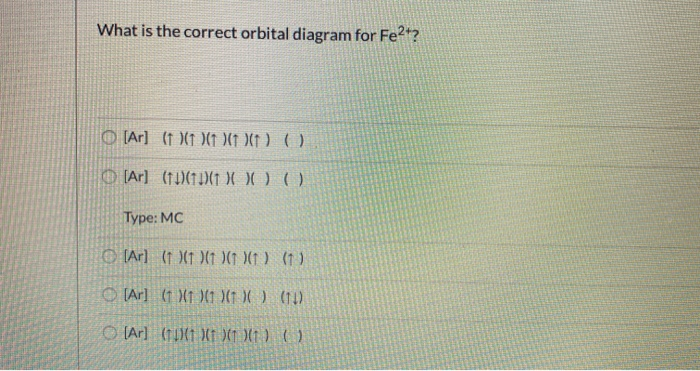
Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ... Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. Draw orbital box diagrams for Fe^2+, Fe^3+, Zn, and Zn^2 ... Answer to: Draw orbital box diagrams for Fe^2+, Fe^3+, Zn, and Zn^2+. Tell which is paramagnetic. [Paramagnetic means that it has unpaired... Solved What is the correct orbital diagram for Fe2 ... This problem has been solved! See the answer. See the answer See the answer done loading. What is the correct orbital diagram for Fe2+? Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Previous question Next question.
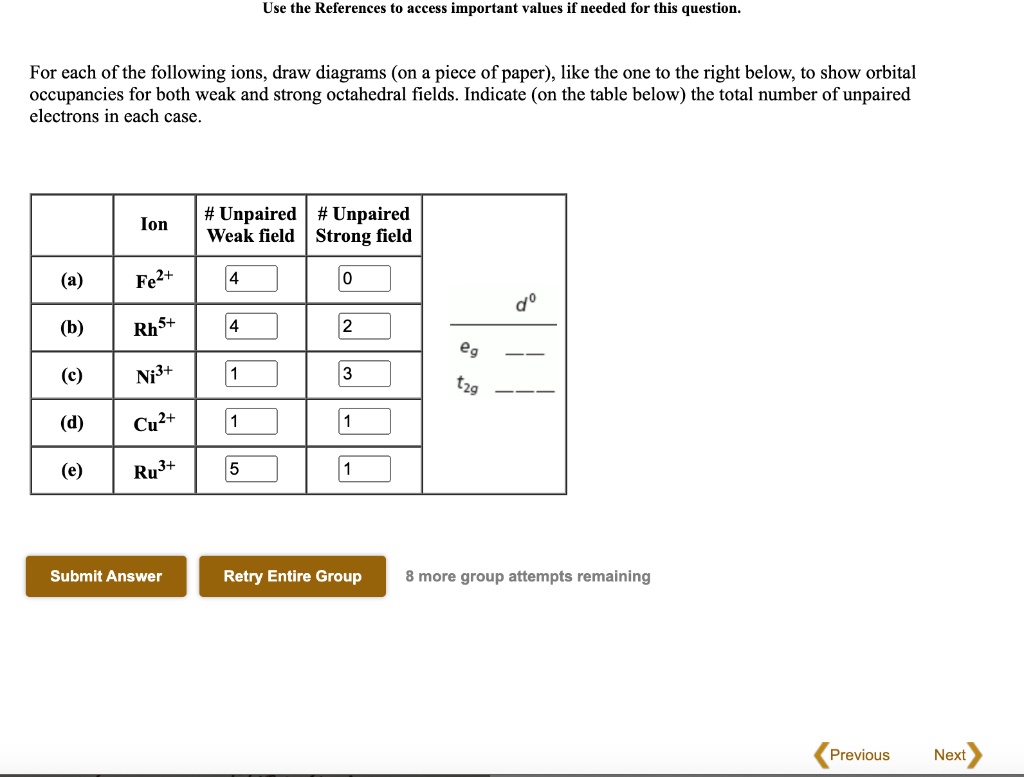
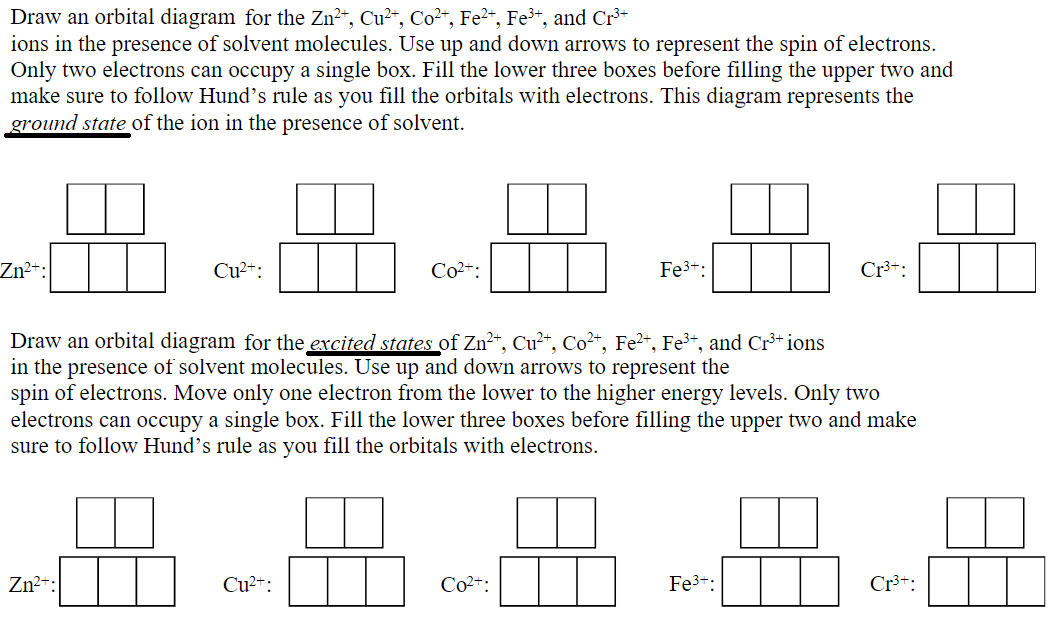
Answered: Draw an orbital diagram with boxes as… | bartleby Solution for Draw an orbital diagram with boxes as shown in Figure 1 for the Zn2+, Cu2+, Co2+, Fe2+, Fe3+, and Cr3+ ions in the absence of solvent molecules.…
Electron Configuration Questions and Answers | Study.com Using an orbital box diagram and noble gas notation, show the electron configuration of the gadolinium(III) ion. ... Write the condensed ground-state electron configuration for Fe2+. View Answer.
40 orbital diagram of fe - Diagram For You Orbital diagram of fe Atomic Orbital Diagram for Iron (Fe) Iron ion (Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron (Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons.
What is the correct electron configuration for zirconium Z ... Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿⇂ ↿⇂ ↿⇂ 4d ↿ ↿ 4f 5s ↿⇂ 5p 5d 5f: ... Fe2+ is easy to oxidize to Fe3+ because ions with an odd charge are most stable for atoms with an even atomic number.
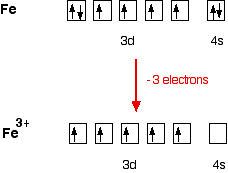
Electron Configurations of Ions - chem-textbook For example, iron (1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2) forms the ion Fe2+ (1s 2 2s 2 2p 6 3s 2 3p 6 3d 6) by the loss of the 4s electron and the ion Fe3+ (1s 2 2s 2 2p 6 3s 2 3p 6 3d 5) by the loss of the 4s electron and one of the 3d electrons. Although the d orbitals of the transition elements are—according to the Aufbau principle—the ...
PDF Molecular orbital (SCF-Xc-SW) theory of Fe2+-Mn3+, Fe3 ... Molecular orbital diagram for the charge configura- tion Fe2*Mn3*O,0. This is not a stable electronic configuration, since an occupied spin-down Fe(tr") orbital lies above an unoc- cupied spin-up Mn(e") orbital. magnitude of the Fe3*-Mn3+ magnetic coupling.
Fe2+ Orbital Diagram - schematron.org The electron configuration for Fe2+ will be 1s2 2s2 2p6 3s2 3p6 4s2 3d4 there is a special stabilty needed to balance the number of electrons in the 3d orbital. can be accommodated in the metal d orbitals. • d0 ions - Ti4+, Zr4+, V5+, Fe2+ , d6.
Iron(Fe) electron configuration and orbital diagram Atomic Orbital Diagram for Iron (Fe) Iron ion (Fe 2+ ,Fe 3+) electron configuration Ground state electron configuration of iron (Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons.
Electron Configuration of Fe2+ and Fe3+ - YouTube The electron configuration for Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6The electron configuration for Fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5Ask me questions: ...
Domain Wall Dynamics in a Ferroelastic Spin Crossover ... Pinned and mobile ferroelastic domain walls are detected in response to mechanical stress in a Mn3+ complex with two-step thermal switching between the spin triplet and spin quintet forms. Single-crystal X-ray diffraction and resonant ultrasound spectroscopy on [MnIII(3,5-diCl-sal2(323))]BPh4 reveal three distinct symmetry-breaking phase transitions in the polar space …
Essentials of Physical Chemistry by B.S. Bahl ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of
Essentials of Physical Chemistry by B.S ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of
A thiolate-bridged FeIVFeIV μ-nitrido complex and its ... Dec 23, 2021 · The N p x and p z orbitals overlap with the in- and out-of-phase combinations of the two Fe d xz orbitals, respectively, and furnish two σ-bonding MOs denoted as Fe1–Fe2–N σ x and Fe1–Fe2 ...
What is the Fe2+ electron configuration? - Quora Answer (1 of 12): The configuration of neutral Fe atom is: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s² where 4s electrons are the most energetic ones, therefore they are the first ones to be removed when atom is ionized. Thus, configuration of Fe⁺ cation is: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s¹ and of Fe²⁺ cation is...
d-orbitals - The bonding, synthesis and electrochemistry ... Orbital 2a"-j ^ has a metal dz^ mixed in with the metal orbital in an antibonding manner and hence is also destabilised relative to [Fe2]^". The other non-bonding orbital, 1 b"^ g is similar to 1a"2y in that it would require a 9 interaction from the sulphurs to obtain a bonding arrangement.
Solid solution - Wikipedia Nomenclature. The IUPAC definition of a solid solution is a "solid in which components are compatible and form a unique phases".. The definition "crystal containing a second constituent which fits into and is distributed in the lattice of the host crystal" given in refs., is not general and, thus, is not recommended.
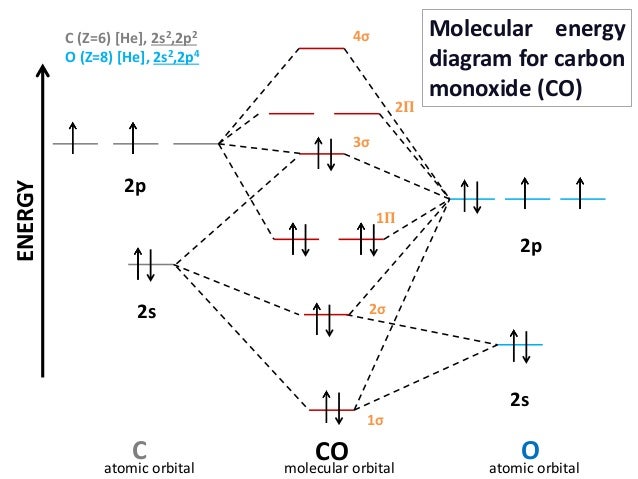
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
electron configuration for Fe2+ - CHEMISTRY COMMUNITY So the electron configuration for Fe is [Ar] 3d^6 4s^2. Fe^2+ means that 2 electrons are taken away. You start removing e- from the outermost shell. The outermost shell, in this case, is the 4s orbital. So removing 2 electrons would leave you with the electron configuration of [Ar] 3d^6. Hope this helps! Top DAllaf Posts: 20
Solved What is the correct orbital diagram for Fe2+? O [Ar ... Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: What is the correct orbital diagram for Fe2+? O [Ar] ( ) ( ) ( ) (x (). [Ar] (11) (1) ( O) ( ) Type: MC [Ar] (1 X (1 ) ( ) ( ) ( ) (1 [Ar] (X1)1 X1 ...
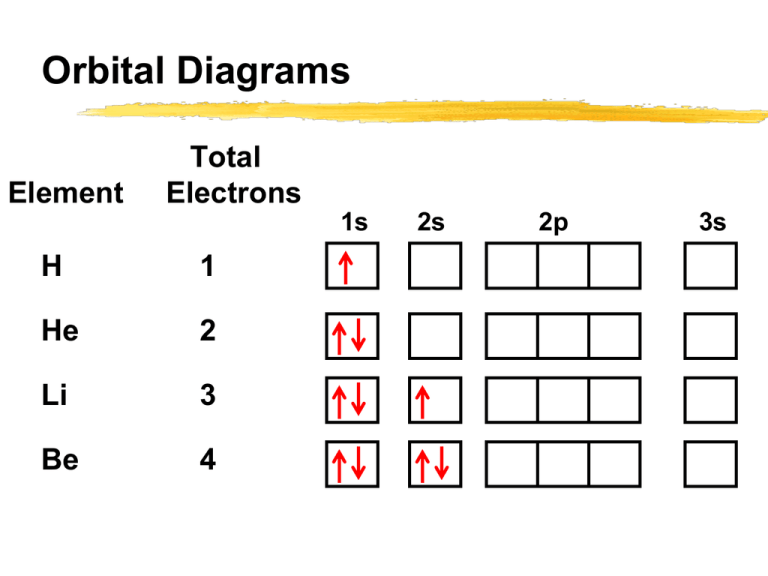
Chemistry Review: Exam 2 Flashcards - Quizlet In an orbital diagram, the arrows are used to indicate the relative spin of an electron (indicative of its ms value). Which of the following correctly describes a violation of the Pauli exclusion principle? ANSWER: a. Arrows in the same orbital with the same direction. b. Arrows in the same subshell with the same direction.
Orbital Diagram For Fe3+ - schematron.org Orbital Diagram For Fe3+. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). Fe3+ loses an electron from the d You take electrons out of the 4s orbital, before you take them out of the 3d orbital, because it is lower. -3,-2,-1,0,1,2,3 c.
40 orbital diagram for fe - Diagram For You fe2+ orbital diagram - wiring diagrams a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) molecular orbital method in particular.electron configuration for fe2+ - chemistry …
(Color online) The upper part shows the MO diagram of an ... Download scientific diagram | (Color online) The upper part shows the MO diagram of an Fe1-O-Fe2 complex inside GFO. The lower part of the figure sketches the corresponding DOS. Marked in red and ...



















0 Response to "42 fe2+ orbital diagram"
Post a Comment