42 lewis dot diagram for nitrogen
What Is The Electron Dot Diagram For Nitrogen ... What are electron dot diagrams used for? Lewis structures (also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Why nitrogen gas is unreactive? it is an unreactive gas. Lewis Structure Questions and Answers | Study.com Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a …
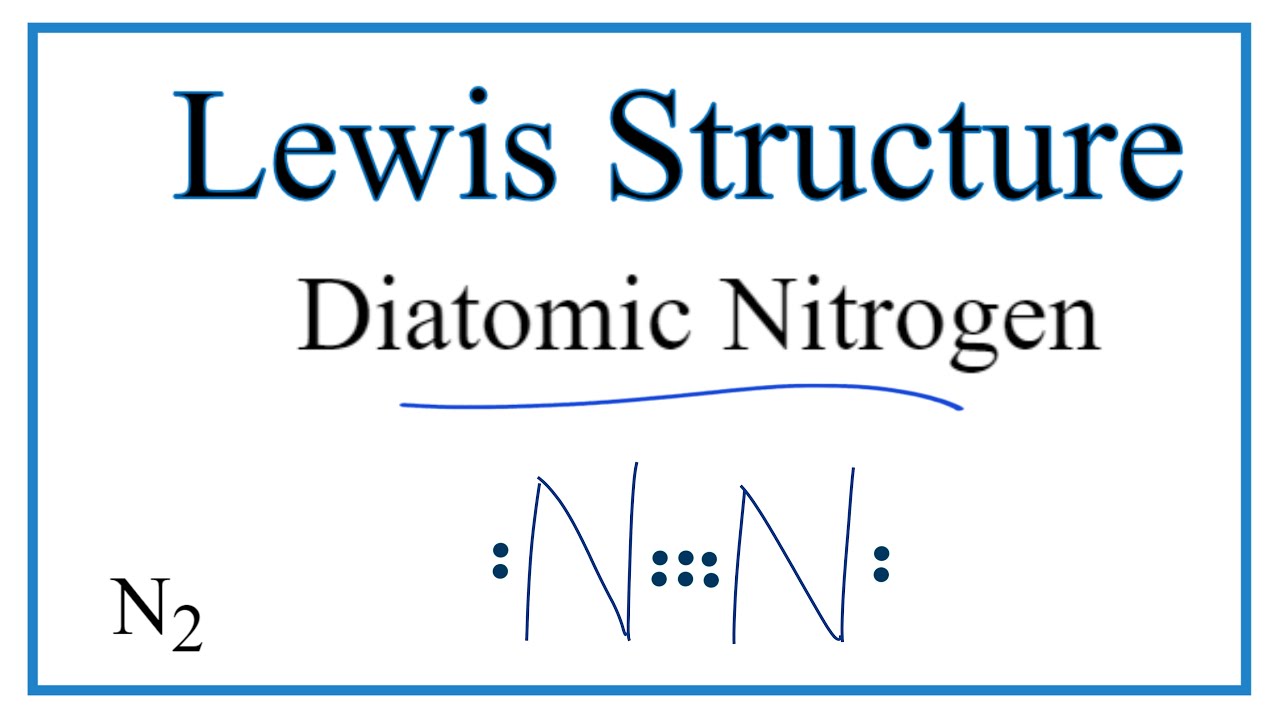
N2 Lewis Structure: How to Draw the Dot Structure for N2| Chemical ... Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, ...

Lewis dot diagram for nitrogen
What is the Lewis dot structure for nitrogen ... The most simple way to determine the hybridization of NO2 is by drawing the Lewis structure and counting the number of bonds and lone electron pairs around the nitrogen atom. You will find that in nitrogen dioxide there are 2 sigma bonds and 1 lone electron pair. What is the hybridization of NO2+? Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ... Lewis structure calculator | Lewis structure generator Lewis structure definition | What is a Lewis dot diagram? The formation of a chemical bond (ionic and covalent) involves the transfer of electrons or the exchange of electrons. Lewis structures can be used to represent valence shell electrons in a chemical bond.
Lewis dot diagram for nitrogen. N2 Lewis Structure: Full Guide (2022 Updated) Nitrogen has five valence electrons in the N2 electron dot structure, classified as a group 5 on the periodic table. Align the two Nitrogens and then sandwich two valence electrons between them to make a chemical bond. There will be no center atom in the Lewis structure since both atoms have the same electronegativity. Nitrogen Triiodide Lewis Structure Nitrogen Triiodide Lewis Structure - There are 24 valence electrons available for the Lewis structure for NO 3-. To determine its Lewis Structure we will sh. Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube . H 2 S NCl 3 OH -. NI3 Lewis Structure Nitrogen Triiodide NI3 is a chemical formula for Nitrogen ... Lewis Dot Structure for Nitrogen Atom (N) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ... NO Lewis Dot Structure | Science Trends March 3, 2020 - Lastly, there is a single unpaired electron on the nitrogen atom. This free radical explains the majority of nitric oxides chemical behavior. A Lewis structure (also called Lewis dot formulas, Lewis dot structures, or electron dot structures) are pictorial diagrams that represent the bonding ...
CN- lewis structure, molecular orbital diagram, and, bond ... Steps to draw the lewis dot structure for CN¯ (Cyanide) 1. Count Valence electrons in CN¯ In the first step, count all valence electrons present in the CN molecule. As carbon belongs to the 14th group in the periodic table, so, it has 4 valence electrons and Nitrogen belongs to the 15th, so, it has 5 valence electrons. What is the Lewis structure of N2? | Socratic October 30, 2015 - In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The ... What is the Lewis dot structure of nitrogen? - MVOrganizing August 20, 2019 - Knowledge Bank: Quick Advice for Everyone · It’s that time of year again, and this exam season only seems to be made more stressful by the fluctuating weather and the covid restrictions that seem to be easing at an excruciating snail’s pace. Being shut indoors with a pile of revision on ... Lewis Dot Structure for Nitrogen Atom (N) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...
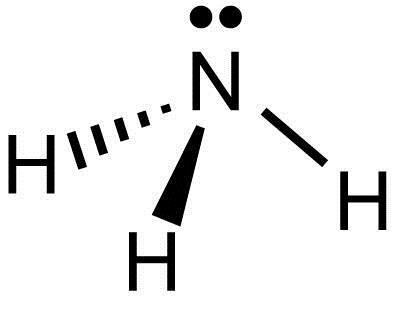
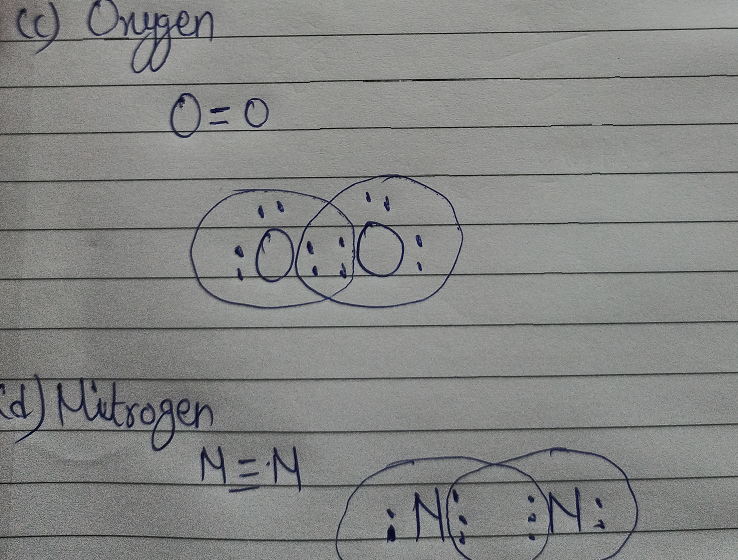
N2 Lewis Structure, Molecular Geometry, and Hybridization ... 18.03.2022 · Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen. Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li NO3 Lewis Structure, Molecular Geometry, and Hybridization 16.03.2022 · Construction of NO3 Lewis Dot Structure. 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. 3. Notice the number of ... 7.3 Lewis Symbols and Structures – Chemistry Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds:
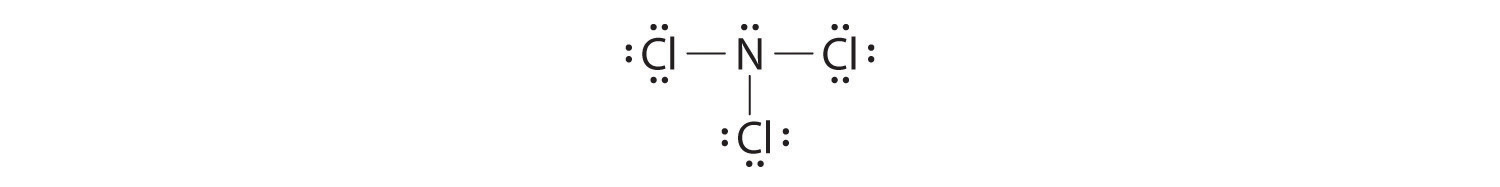
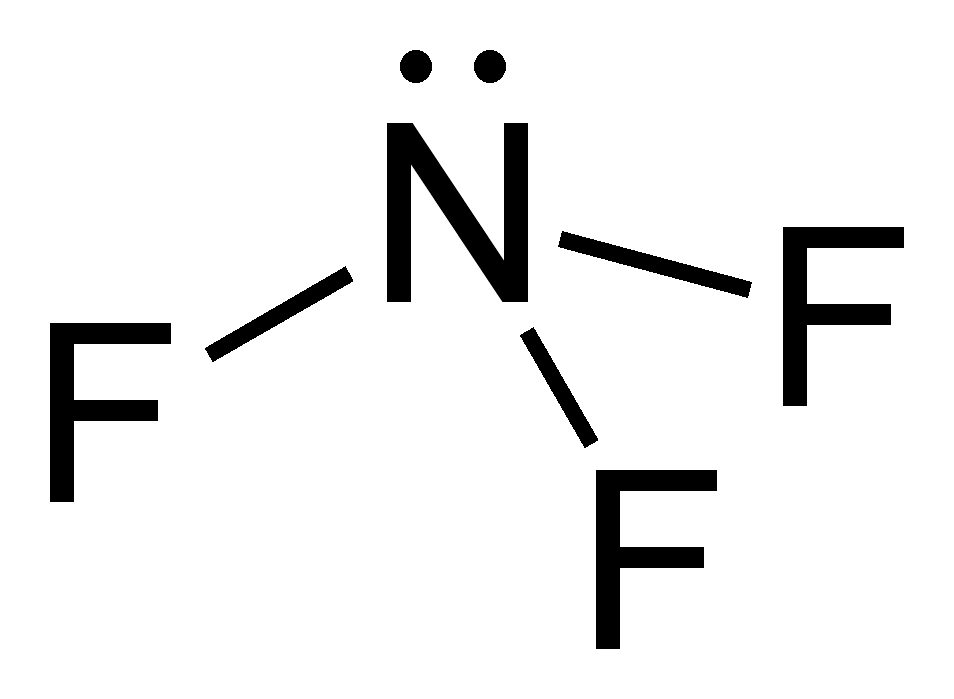
Nitrogen trifluoride (NF3) lewis dot structure, molecular ... So, nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the NF3 lewis dot structure. Hence the formula of NF3 becomes AX 3 N 1 So, according to the VSEPR chart , if the molecule has the formula of AX 3 N 1 , it has a molecular shape of trigonal pyramid and electron geometry of tetrahedral.
Lewis Structure for NO2 (Dinitrogen or Nitrogen Gas) Lewis Structures for NO2. Step-by-step tutorial for drawing the Lewis Structure for NO2.
NF3 (Nitrogen trifluoride) Lewis Structure - Steps of Drawing NF 3 (Nitrogen trifluoride) Lewis Structure | Steps of Drawing In the lewis structure of Nitrogen trifluoride (NF 3), there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps.
What Is The Lewis Dot Structure Of Nitrogen? Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom. Single bonds are represented by a pair of dots or one line between atoms.
Nitrogen University of Colorado Denver, located in Denver, CO offers more than 112 degrees in 8 schools, educating people who are serious about their future!
Lewis dot structures One of the pairs of electrons on the nitrogen of ammonia is not bonded. We call that a lone pair. It occupies its own stable orbital, as shown in the stick diagram on the right. Lewis structures tell us about the most-likely bonding arrangement and bond types of molecules, but they tell us ...
Lewis Dot Diagram - Organic Chemistry - Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
Lewis Dot Diagram For N2 - schematron.org on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use .
How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
Lewis Structure for N2 (Dinitrogen or Nitrogen Gas) Lewis Structures for N2. Step-by-step tutorial for drawing the Lewis Structure for N2.
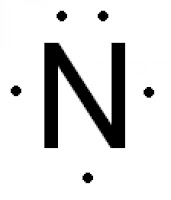
What is the electron dot diagram for nitrogen? Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). Click to see full answer Also know, what are electron dot diagrams used for?
HCN Lewis Structure: How to Draw the Dot Structure for HCN ... Transcript: For the HCN Lewis structure we have one valence electron for Hydrogen, we have four for Carbon, and we have five for Nitrogen, for a total of ten valence electrons for the HCN Lewis structure. We'll put the Carbon in the center, because it's less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures.
Lewis Structures: Single, Double & Triple Bonds - Video ... 21.11.2021 · Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compounds. Learn how to represent single, double and triple bonds with lines instead of dots.
Lewis Dot Structure - Easy Hard Science December 28, 2021 - Lewis Dot Structure: Easy instructions, complete examples, and a printable worksheet to master the art of drawing molecules.
Nitrogen triiodide (NI3) lewis dot structure, molecular ... Nitrogen triiodide (NI3) lewis dot structure, molecular geometry, ... The element which repeats least in the compound should be the central atom in the lewis diagram. So, in the case of NI3, the nitrogen atom is repeated only one time whereas the iodine atom repeats three times.
Draw and explain the Lewis dot structure of nitrogen ... The Lewis dot structure of Nitrogen is shown below. This shows 5 dots around the symbol of nitrogen. These five dots represent the five electrons that... See full answer below. Become a member and...
beryllium and nitrogen lewis dot structure - Intro Property beryllium and nitrogen lewis dot structure. Lewis Structures are important to learn because they help us predict: the shape of a molecule. Step 1: Determine the total number of electrons available for bonding. Nitrogen (IV) oxide (NO 2) is a well-known example. Electron dots are typically arranged in four pairs located on the four "sides" of ...
How do you write/draw a Lewis structure for N? | Socratic April 25, 2016 - The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration of Nitrogen is 1s^2 2s^2 2p^3 The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.
What is Lewis dot diagram of nitrogen gas? - Answers The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . .
What is the electron dot diagram for nitrogen? – Rehabilitat... Electron Dot Diagrams lithium 1s22s1 1 valence electron beryllium 1s22s2 2 valence electrons nitrogen 1s22s22p3 5 valence electrons neon 1s22s22p6 8 valence electrons Which is the correct Lewis dot diagram for nitrogen? The five dot represent the five valence electrons of nitrogen.
Lewis Dot Structures - Definition and Example | Chemistry Lewis dot structure is also known by some other names such as electron dot structure, Lewis electron-dot formula, or Lewis Electron Dot Structures (LEDs), etc. It has many applications in Chemistry, especially for the topics related to the study of bonds between atoms, the atomic structure of atoms, molecular & organic Chemistry, etc.
NO2 Lewis Structure: Complete Guide (2022 Updated) The nitrogen atom is in the center, surrounded by Oxygen atoms. We'll now use dot structures to encircle the electrons with valence electrons. There are 17 valence electrons in the Lewis structure of NO2 - with an unpaired electron that is not stable because of the odd number of electrons.
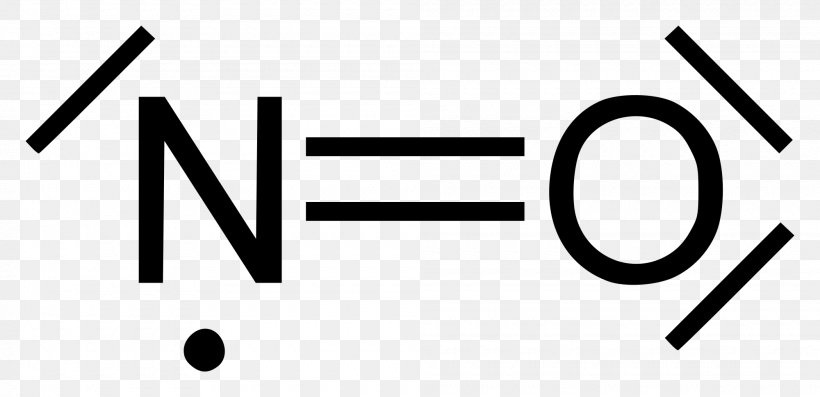
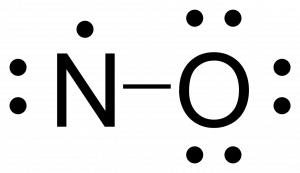
Lewis dot of Nitrogen Monoxide (NO) | Chemistry Net what is the Lewis structure of NO nitrogen monoxide, Lewis structures of nitrogen monoxide, Lewis electron dot structures of nitrogen monoxide, electron dot structures of nitrogen monoxide, NO Lewis structures, NO electron dot structures, NO dot structures, pi an d, for the draw, lewis no, dot structure of NO, electron dot lewis structure of NO, resonance structures of NO, ap chemistry lewis ...
Nitrogen trichloride (NCl3) lewis dot structure, molecular ... Lewis diagram is a representation of how electrons are arranged around individual atoms in a structure. NCl3 lewis structure is the same as the NF3 structure. It contains one nitrogen atom at the center and three chlorine atoms spaced evenly around it.
lewis dot diagram Quiz - Quizizz This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
Nitrogen (N2) Molecule Lewis Structure Nitrogen (N 2) Molecule Lewis Structure Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure
What is the Lewis Structure for N2 (nitrogen gas)? - Quora December 31, 2015 - Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron...
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
OneClass: What is the Lewis structure of Nitrogen? May 23, 2019 - Get the detailed answer: What is the Lewis structure of Nitrogen?
Lewis Electron Dot Diagrams – Introductory Chemistry ... For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron …
What is the Lewis dot structure for nitrogen gas ... What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
NO2 (Nitrogen Dioxide) Lewis Dot Structure | Science Trends Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.It is slightly toxic to humans, on account of its tendency to react in the human body and produce …
Electron Dot Diagrams | Chemistry for Non-Majors When examining chemical bonding, it is necessary to keep track of the valence electrons of each atom. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would ...
N2 Lewis Structure - Easy Hard Science - Learn With Dr ... January 17, 2022 - The two letter N’s in the N2 Lewis structure represent the nuclei (centers) of the nitrogen atoms. The nuclei contain the protons and neutrons, which are the solid parts of the molecule. Interestingly, the dots and lines represent electrons, which are not solid. The diagram is drastically ...
Lewis structure calculator | Lewis structure generator Lewis structure definition | What is a Lewis dot diagram? The formation of a chemical bond (ionic and covalent) involves the transfer of electrons or the exchange of electrons. Lewis structures can be used to represent valence shell electrons in a chemical bond.
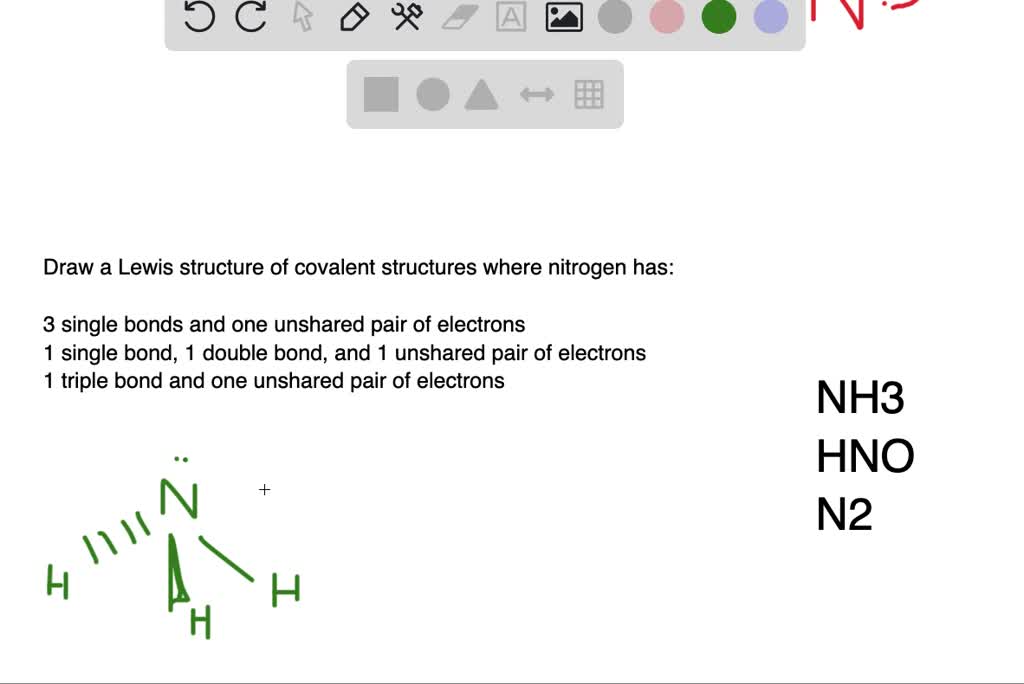
draw a lewis structure of a covalent compound in which nitrogen has a three single bonds and one u 3
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ...
What is the Lewis dot structure for nitrogen ... The most simple way to determine the hybridization of NO2 is by drawing the Lewis structure and counting the number of bonds and lone electron pairs around the nitrogen atom. You will find that in nitrogen dioxide there are 2 sigma bonds and 1 lone electron pair. What is the hybridization of NO2+?
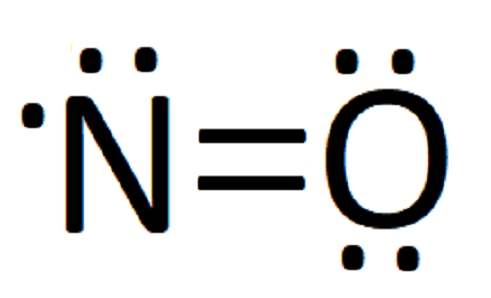







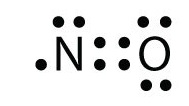

















0 Response to "42 lewis dot diagram for nitrogen"
Post a Comment