39 energy diagram for exothermic reaction
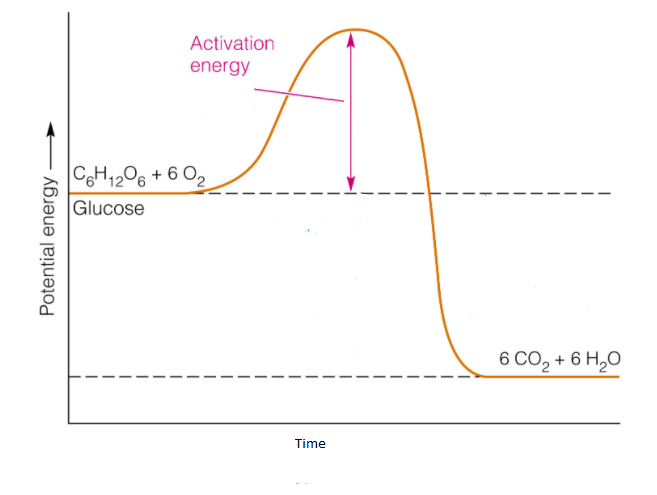
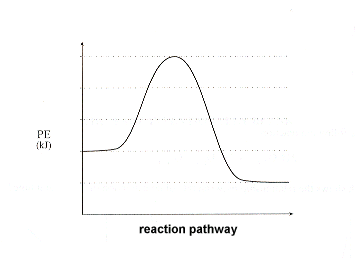
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. Creative Diagram Of Exothermic Reaction - Glaucoma Template You can start with a generic potential energy diagram for an exothermic reaction. A The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. There is a greater difference in energy between the reactants and products. Because the reactants have more energy than the products they are less stable.
› questions › 18837251. which type of chemical reaction results in the absorption ... Feb 03, 2022 · 1. which type of chemical reaction results in the absorption of energy? a.exothermic b.combustion c.replacement d.endothermic 2. which type of chemical reaction occurs when natural gas is burned? 1. replacement 2. endothermic 3. decomposition 4. exothermic 3. which part of an energy graph is represented by the highest point? 1. the products of the reaction 2. the activated state 3. the amount ...
Energy diagram for exothermic reaction
1- Use the reaction to complete the sentence. 2CO + O2 ... 10-02-2022 · 1- Use the reaction to complete the sentence. 2CO + O2 → 2CO2 Carbon monoxide and oxygen combine to produce carbon dioxide. The total bond energy of the products is 1,472 kJ. The bond energy of each carbon-oxygen bond in carbon dioxide is _____. (1 point) a 368 kJ b 1,472 kJ c 736 kJ d 2,944 kJ 2-Use the table to answer the question. Reaction CH4 + … How do you know if a diagram is endothermic or exothermic ... The energy level decreases in an exothermic reaction. The energy level increases in an endothermic reaction. The overall change in energy in a reaction is the difference between the energy of the reactants and products. The diagram shows a reaction profile for an exothermic reaction. Energy Diagrams | OpenOChem Learn Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0).
Energy diagram for exothermic reaction. PDF Exhothermic and Endothermic Reactions / Activation Energy ... least the two following ways. The first of these is (A) by using an Enthalpy or Energy Profile diagram, and you must be able to differentiate between Exhothermic and Endothermic processes, and the second required way is (B) to manipulate E a in a Maxwell-Boltzmann diagram. A) Enthalpy or Energy Profile diagrams Welcome to CK-12 Foundation | CK-12 Foundation FlexBook Platform®, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation. Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ... PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... diagram. The vertical axis on this diagram represents the energy level and the horizontal axis represents the progress of the reaction from reactants to products. Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between
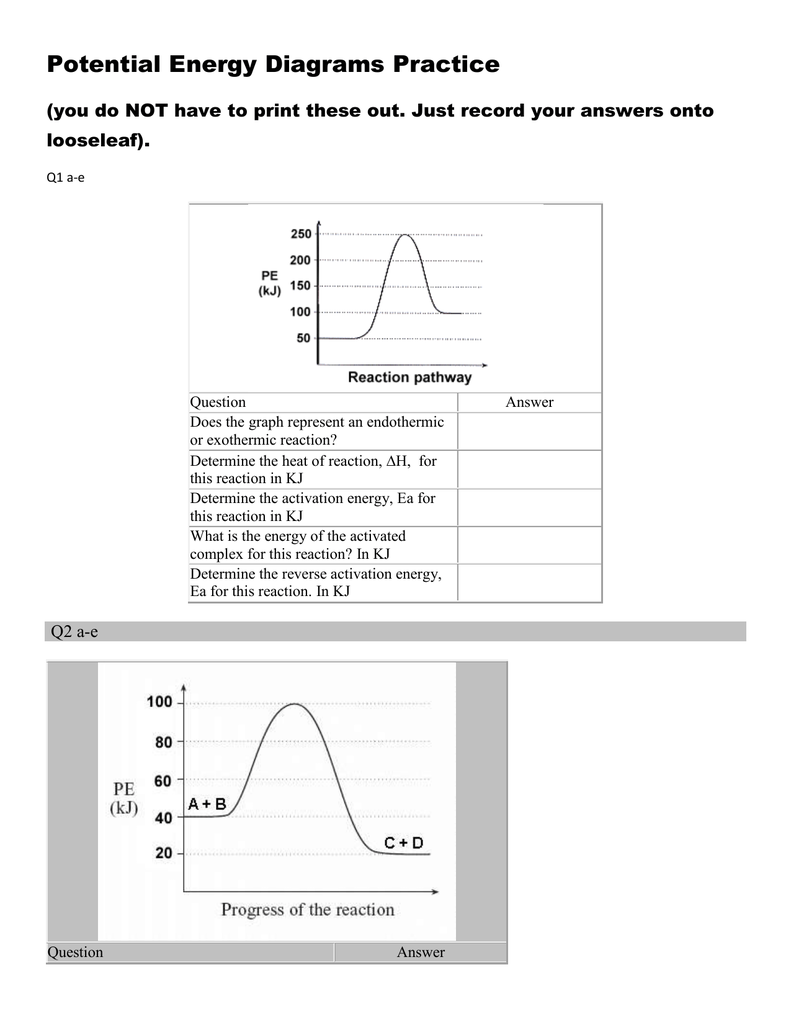
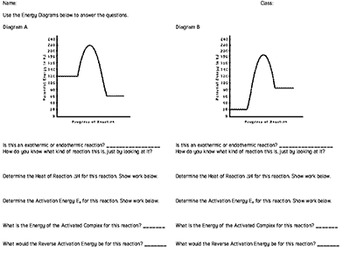
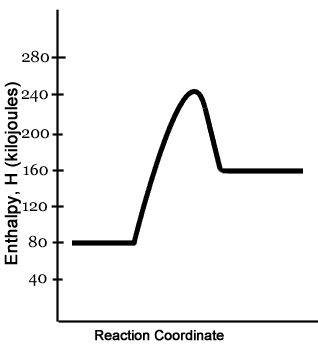
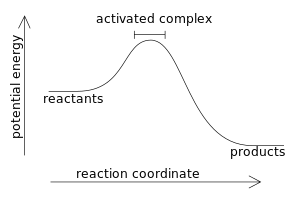
PDF NAME Kinetics Potential Energy Diagrams - ISD 622 Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products ... (Get Answer) - Consider the reaction diagram. This ... Draw an energy level diagram for a reaction in which the total energy of the reactants is 50 kJ mol-1, the total energy of products is 120 kJ mol-1 and the activation energy for the forward reaction is 120 kJ mol-1. Label the diagram clearly .Is this... Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram. Gibbs Free Energy - Purdue University Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > …
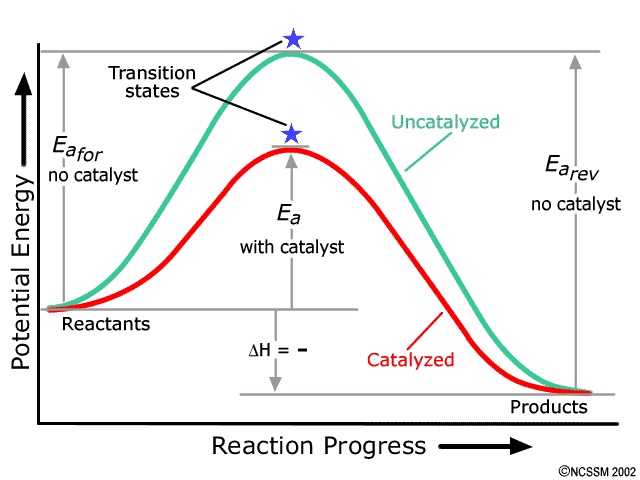
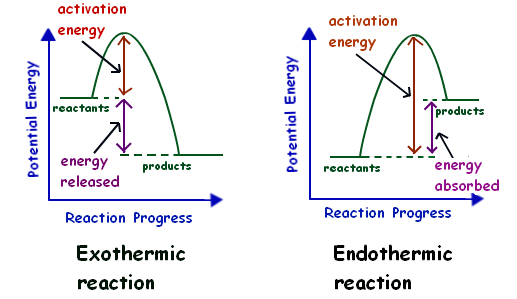
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Potential Energy Diagrams - Kentchemistry.com Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. What are Exothermic Reactions? (with Examples and Video) An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative. Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Energy Changes in Chemical reactions for KS3 Science Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. 10.What are the units for enthalpy change, ΔH
Energy Diagrams of Reactions | Fiveable Exothermic Reactions In the graph for an exothermic reaction, you can see that the products have a lower potential energy, implying that energy has been released from the system. This further proves that ΔH is negative in an exothermic reaction. Image Courtesy of SilaVula Endothermic Reactions
3:05 (Triple only) draw and explain energy level diagrams ... In an exothermic reaction, the reactants have more energy than the products. Energy is given out in the form of heat which warms the surroundings. ΔH is given a negative sign, because the reactants are losing energy as heat, e.g ΔH = -211 kJ/mol. In an endothermic reaction, the reactants have less energy than the products.
› questions › 1871848Which result occurs during an exothermic reaction? A.Light is ... Sep 30, 2021 · What is the total energy of the reaction? Is this an endothermic or exothermic reaction? A.When producing hydrogen iodide, the energy of the reactants is 581 kJ/mol, and the energy of the products is 590 kJ/mol. The equation is shown. H2 + I2 → 2HI A.–9 kJ/mol, endothermic B.9 kJ/mol, endothermic C.9 kJ/mol, exothermic D.9 kJ/mol, exothermic
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
1.4 Energetics - chemrevise 01-04-2020 · Energy: EA ∆H Progress of Reaction Energy reactants Activation Energy: EA ∆H Progress of Reaction Energy Common oxidation exothermic processes are the combustion of fuels and the oxidation of carbohydrates such as glucose in respiration. 1.4 Energetics Definition: Enthalpy change is the amount of heat energy
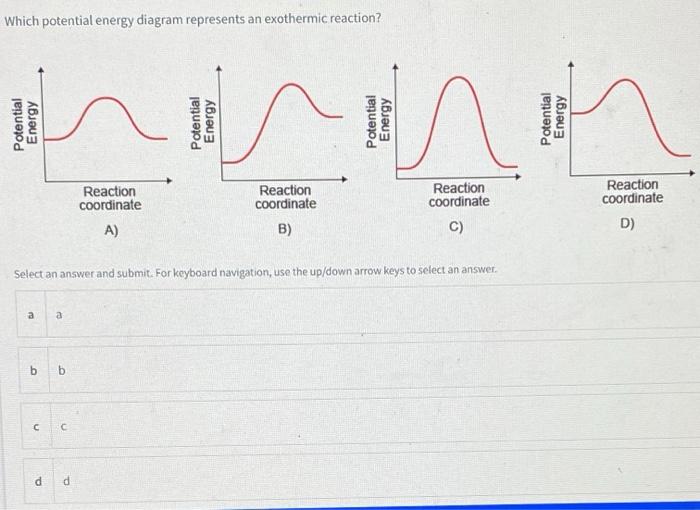
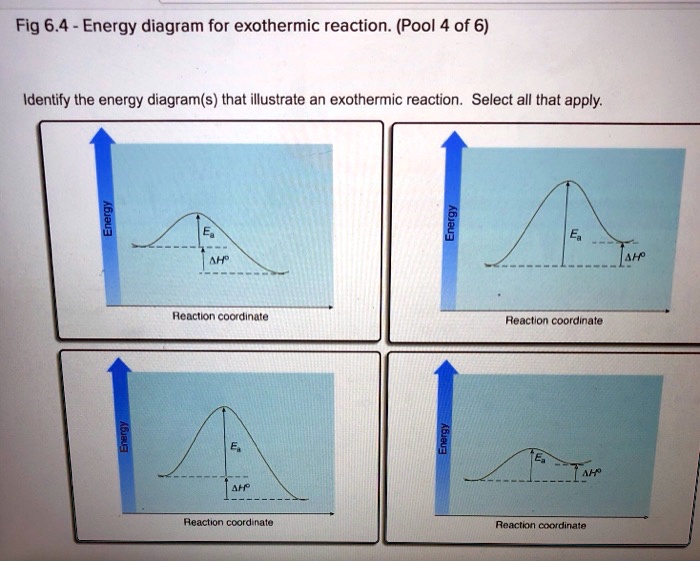
Solved Fig 6.4-Energy diagram for exothermic reaction ... Question: Fig 6.4-Energy diagram for exothermic reaction. (Pool 4 of 6) Identify the energy diagram(s) that illustrate an exothermic reaction. Select all that apply Ea AHO Reaction coordinate Reaction coordinate AHo Reaction coordinate Reaction coordinate . This problem has been solved!
How can I draw a simple energy profile for an exothermic ... You can start with a generic potential energy diagram for an exothermic reaction. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.
How does the energy level diagram show this reaction is ... Energy profile diagrams for endothermic and exothermic reactions Every chemical substance has a certain amount of chemical energy. This energy is given the symbol H and is different for different substances. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured.
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In exothermic reactions, there is more energy in the reactants than ...
Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
What is Enthalpy? - Definition, Endothermic & Exothermic ... A heat absorption reaction is endothermic. Its enthalpy will be positive, and its surroundings will cool down. This reaction (negative enthalpy, heat release) is exothermic. When the reaction happens, due to the gain in heat the device emits, the atmosphere may rise in temperature.
PDF Potential Energy Diagrams the diagrams graphically compare reactant energy to product energy as reaction occurs if reactants have more energy then products, the reaction is exothermic (loss of energy) if reactants have less energy then products, the reaction is endothermic (gain of energy)
Energy level diagrams - Why are there energy changes in ... The reaction shown by the second diagram is more exothermic. There is a greater difference in energy between the reactants and products. The green arrow is longer.
How can I represent an endothermic reaction in a potential ... 20 Dec 2015 — An endothermic reaction must have a positive change in enthalpy. That is, ΔH>0 . This means that the system absorbs heat.1 answer · An endothermic reaction must have a positive change in enthalpy. That is, ΔH>0. This means that the system absorbs heat. ...
Energy Profiles (Energy Diagrams) Chemistry Tutorial Energy Profile for Exothermic Reactions The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1
Representing endothermic and exothermic processes using ... Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
Exothermic and Endothermic Processes | Introduction to ... Exothermic reaction In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings. Endothermic Reactions. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the ...
EXOTHERMIC & ENDOTHERMIC REACTIONS: ENERGY ... ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages
Exothermic Reaction - Definition and Examples | Properties ... Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction.
3.9: Energy in Chemical Reactions - Biology LibreTexts 04-09-2021 · Figure \(\PageIndex{5}\): This diagram of activation energy shows the reactants on the far left and the products on the right. Notice that the reactants hare at a higher energy level than the products; so this reaction releases energy overall. But the reaction consumes energy to get started - this is the activation energy for the reaction.
Energy Diagrams | OpenOChem Learn Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0).
How do you know if a diagram is endothermic or exothermic ... The energy level decreases in an exothermic reaction. The energy level increases in an endothermic reaction. The overall change in energy in a reaction is the difference between the energy of the reactants and products. The diagram shows a reaction profile for an exothermic reaction.
1- Use the reaction to complete the sentence. 2CO + O2 ... 10-02-2022 · 1- Use the reaction to complete the sentence. 2CO + O2 → 2CO2 Carbon monoxide and oxygen combine to produce carbon dioxide. The total bond energy of the products is 1,472 kJ. The bond energy of each carbon-oxygen bond in carbon dioxide is _____. (1 point) a 368 kJ b 1,472 kJ c 736 kJ d 2,944 kJ 2-Use the table to answer the question. Reaction CH4 + …

















0 Response to "39 energy diagram for exothermic reaction"
Post a Comment