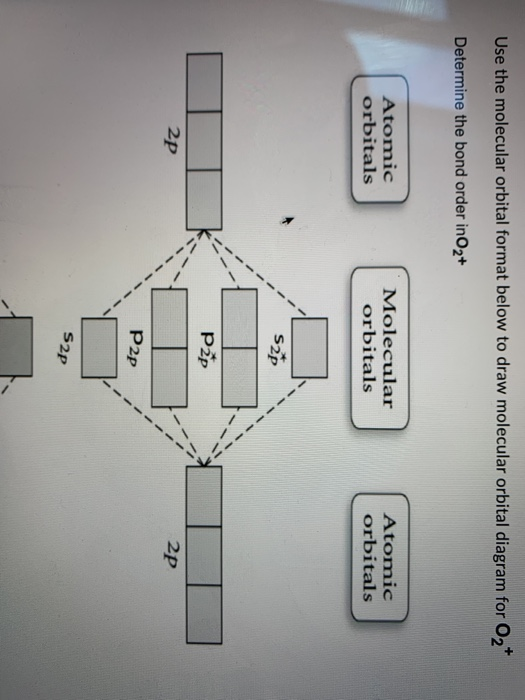
40 o2+ molecular orbital diagram
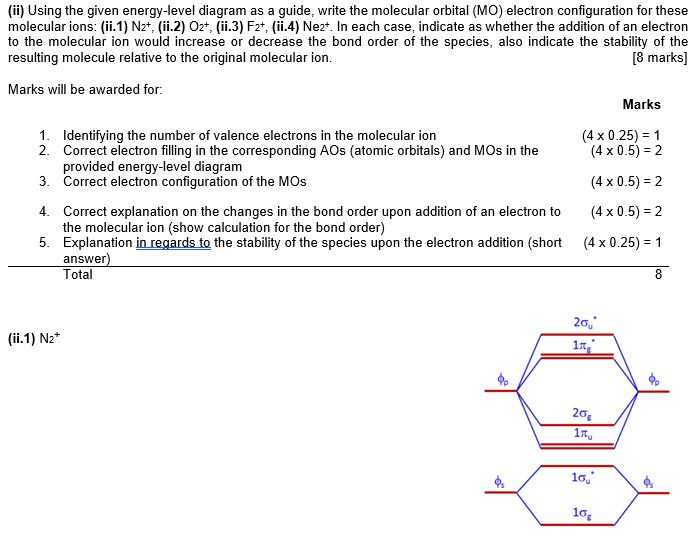
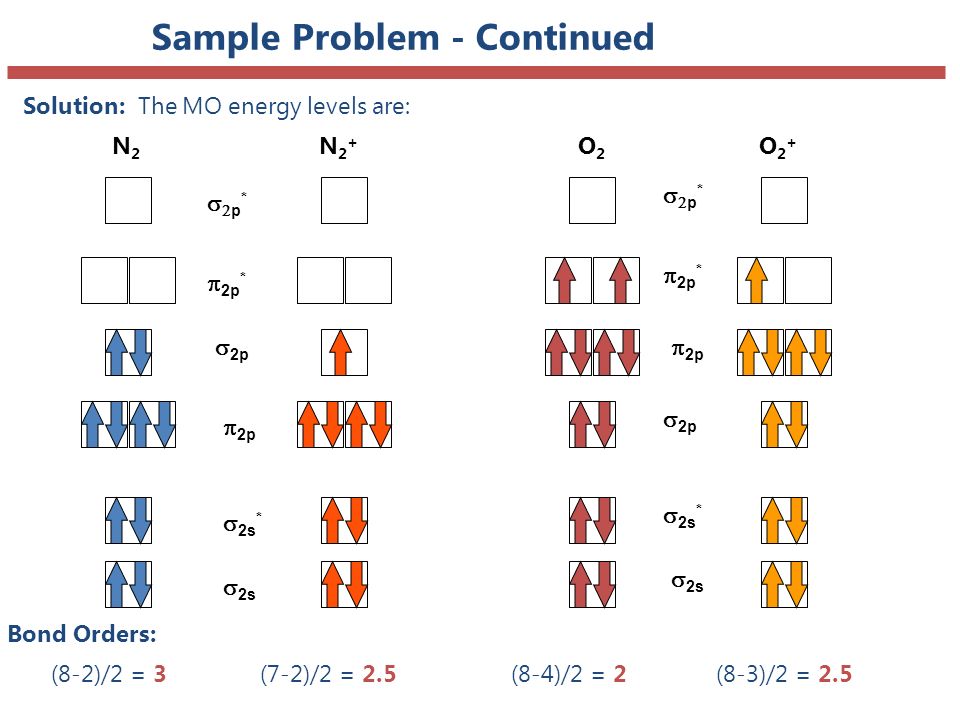
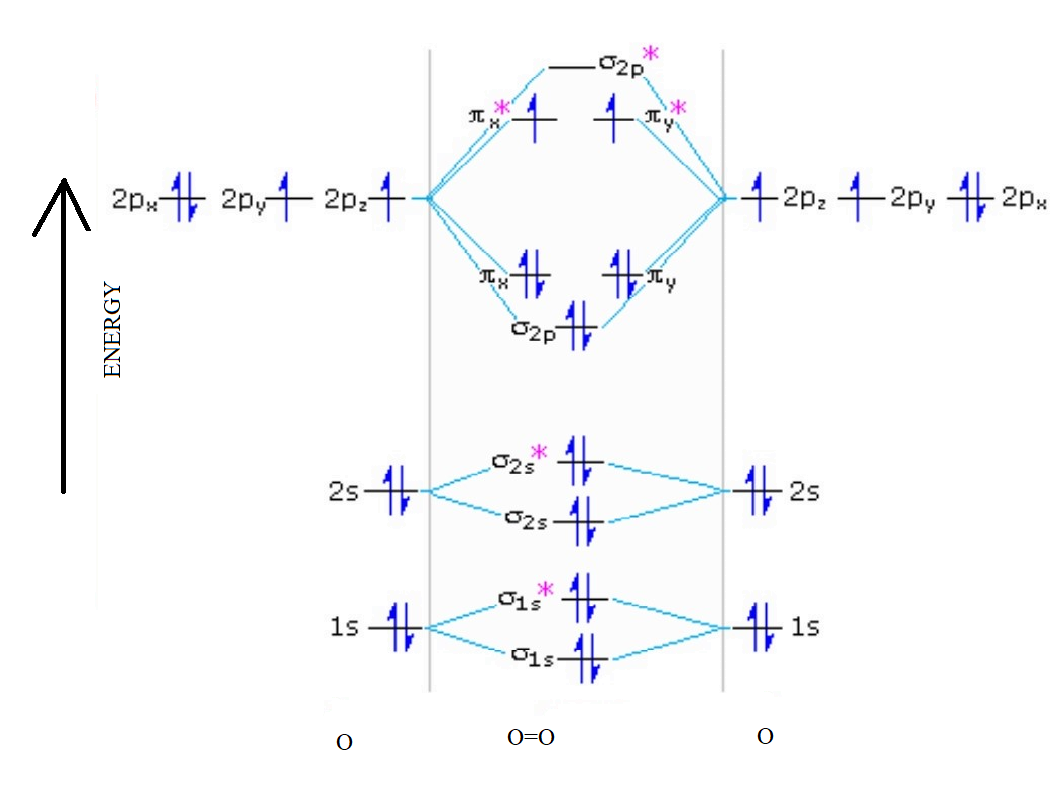
Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Medium Solution Verified by Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b −N a ]/2=[10−6]/2=2. MOLECULAR ORBITAL DIAGRAM OF O2, 02+AND O2(2-). - YouTube In this video, you will study about Molecular Orbital diagram of O2, O2+, O2(2-). We will also calculate the Bond order in each case and also the magnetic be...
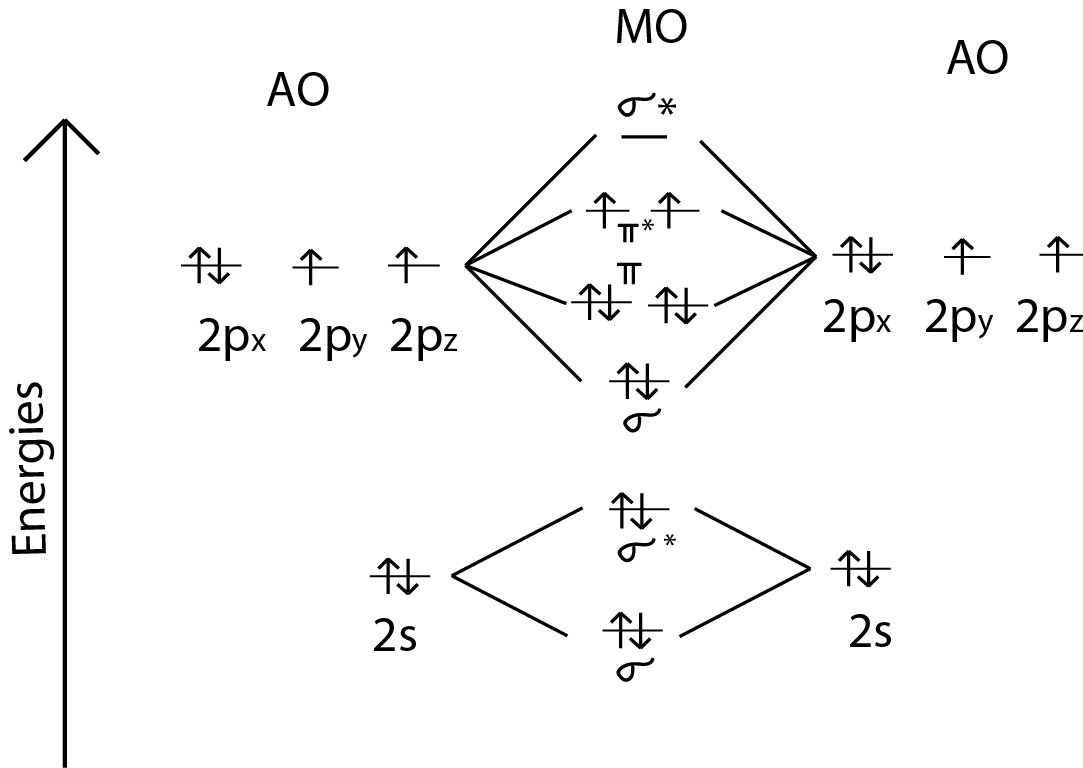
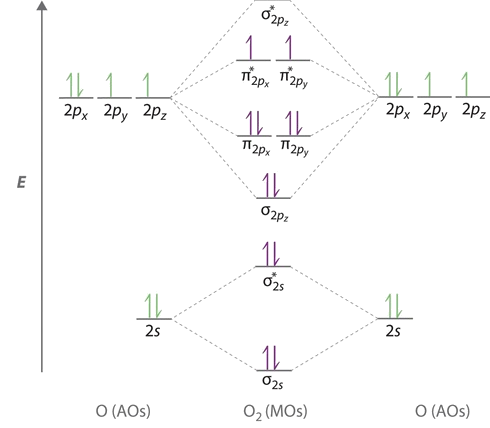
Explain the formation of O2 molecule using molecular class 11 ... The molecular orbital diagram of an Oxygen molecule is as – From the diagram, the electronic configuration of oxygen molecule can be written as - ${O_2}$ :- $\sigma 1{s^2}{\sigma ^*}1{s^2}\sigma 2{s^2}{\sigma ^*}2{s^2}\sigma 2{p^2}\Pi 2p_x^2\Pi 2p_y^2{\Pi ^*}2p_x^1{\Pi ^*}2p_y^1$
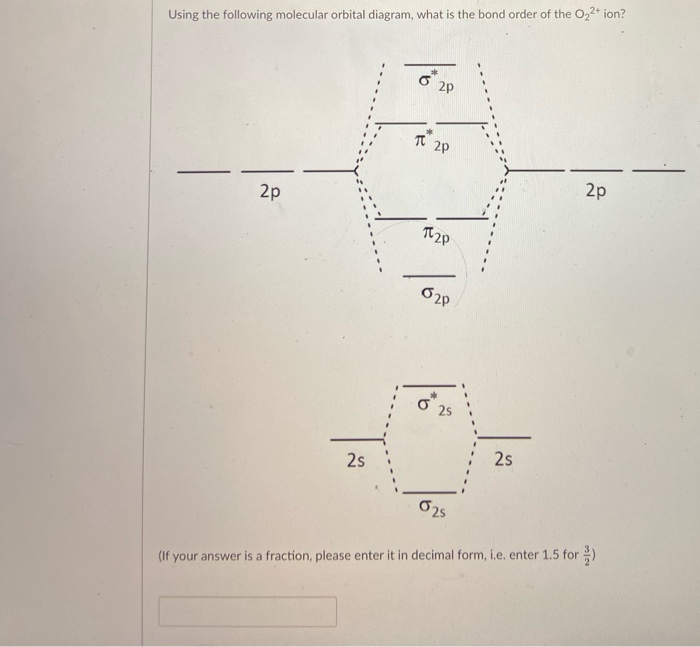
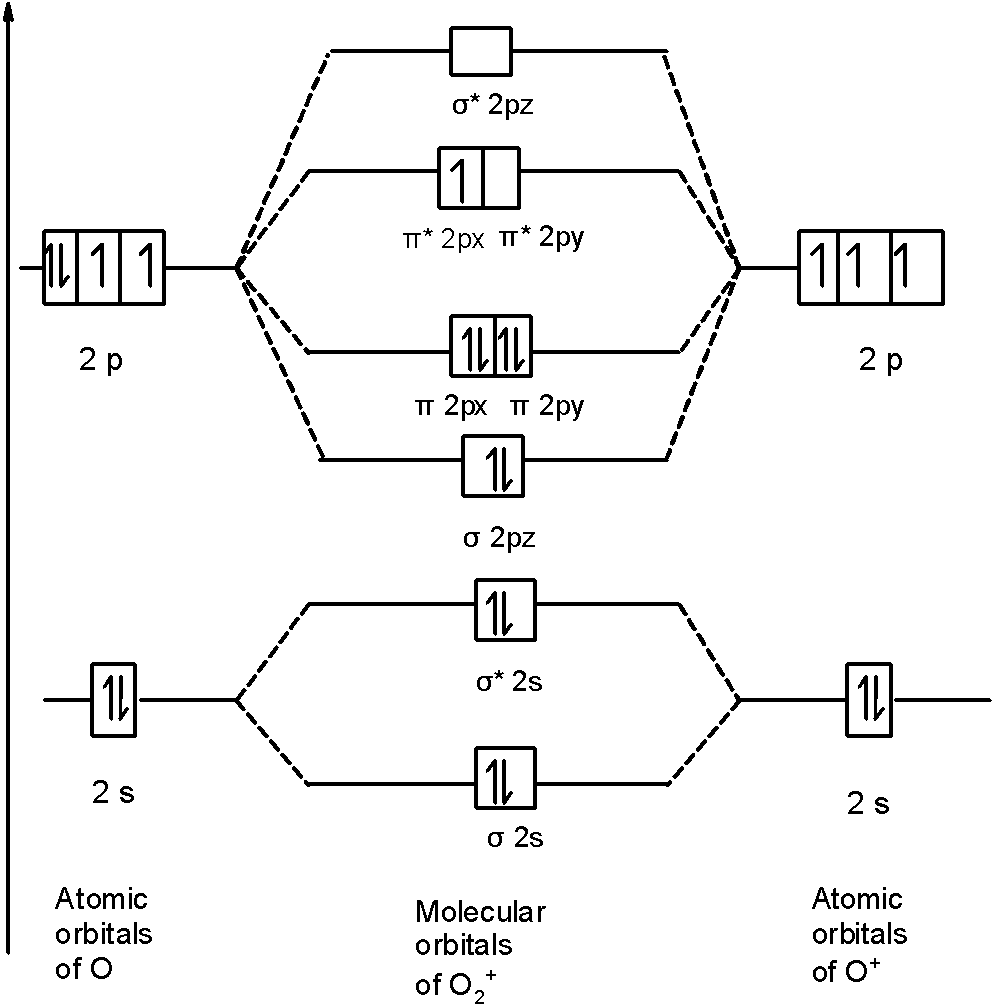
O2+ molecular orbital diagram
8 - Drawing Molecular Orbital Diagrams — Flux Science The way these bonds are placed on any molecular orbital diagram is according to how the atomic orbitals that make the MOs mix. In that mixing, there are two factors to consider: (1) atomic symmetry and (2) mixing. Image via Chegg Symmetry As a bond occurs, a bond or internuclear axis - the line that connects the nuclei of two bonded atoms - forms. Electronic valence molecular orbital configuration of "O ... You'll need the molecular orbital (MO) diagram of O2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get O2. Two 2s orbitals combine to give a σ2s bonding and σ* 2s antibonding MO. Molecular Orbital (MO) Diagram for O2(-) - YouTube When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the othe...
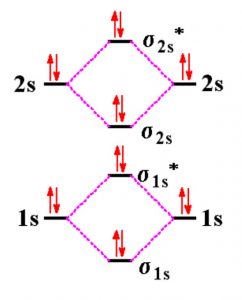
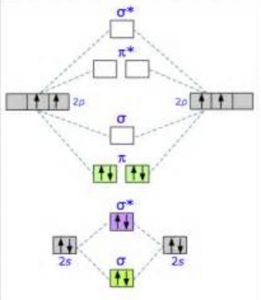
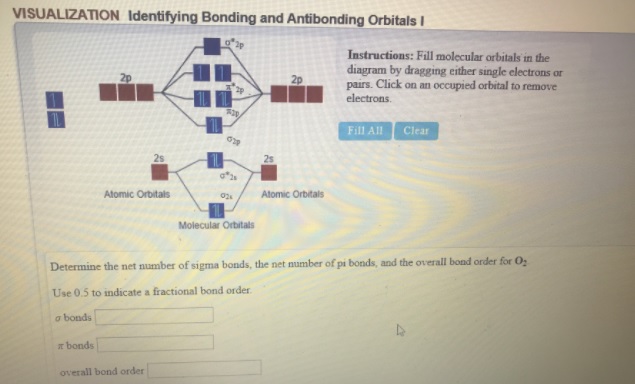
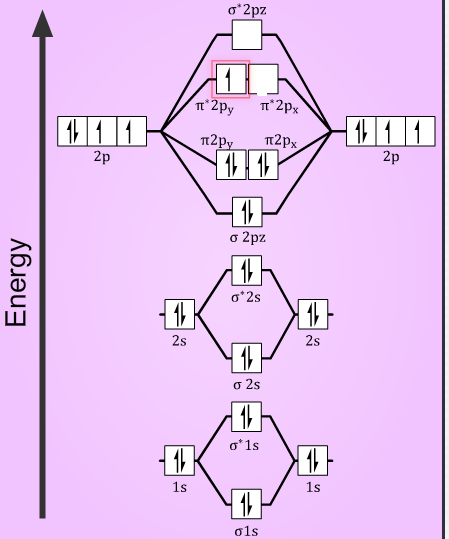
O2+ molecular orbital diagram. 10. Hybridized & Molecular Orbitals; Paramagnetism ... Orbitals split into bonding orbitals (lower) and antibonding orbitals (higher). Electrons fill from lowest energy up. Types of bonds: sigma = no nodal plane separates nuclei; pi = a nodal plane separates nuclei; Paramagnetism: from unpaired electrons in molecular orbitals e.g. liquid oxygen is paramagnetic - can be held by a magnetic field ... Molecular Orbital Theory - Purdue University are combined. The molecular orbital diagram for an O2molecule would therefore ignore the 1selectrons on both oxygen atoms and concentrate on the interactions between the 2sand 2pvalence orbitals. Molecular Orbitals of the Second Energy Level The 2sorbitals on one atom combine with the 2sorbitals on another to form a 2sbonding and a 2s* How many molecular orbitals are there in O2? - Quora Answer (1 of 5): Infinite. Which is the same answer to all questions about numbers of orbitals without constraining qualifiers. What you probably meant to ask is what are the number of (partially?) filled, ground state orbitals: (image from 9.10: Molecular Orbital Theory Predicts that Molecular... PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
More stable among O2+ and O2 - NextGurukul May 19, 2014 — Because According to molecular orbital theory O2+ has 15 electrons &it has one electron in antibonding orbital. molecular orbital diagram of ... Molecular Orbital Diagram of O2, F2, and Ne2 Molecules ... 0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d... What Is The Molecular Orbital Diagram Of O2 ... Dec 18, 2021 · Oxygen is found naturally as a molecule. Two oxygen atoms strongly bind together with a covalent double bond to form dioxygen or O2. Oxygen is normally found as a molecule. It is called dioxygen. What is the bond order of O2 -? 2 O2 has two unpaired electrons in its π* orbitals, and a bond order of 2. How do you draw a molecular orbital diagram for O2? Explain the formation of O2 molecule using molecular ... The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2N b −N a = 28−4 =2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. The last two electrons in p 2px∙ and p 2py∙ orbitals will remain unpaired.
Molecular Orbital (MO) Diagram for O2(2-) - YouTube This is the peroxide ion, O2(2-), so you KNOW it's going to be stable.It has a bond order of 1, which also makes sense. Draw the Lewis diagram of hydrogen pe... 7.7 Molecular Orbital Theory - Chemistry Fundamentals molecular orbital diagram ( Figure 7.7.9 ). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center. MO DIAGRAM O2+ , O2 2+ ,O2- ,O2 2- (preparation of gate ... Follow me on instagram- me on facebook page- ... In molecular orbital diagram for O2+ ion the highest class ... Hint: Molecular orbital theory was put forward by Hund and Mulliken, which can be applied to explain the properties, that was not explained by Valence bond theory. This theory explained the paramagnetic nature of O 2 + ion as per Valence bond theory it should be diamagnetic. - Molecular orbital diagram is the diagrammatic representation of all ...
How many molecular orbitals are in o2 ... O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals.
Molecular Orbital Theory & Bonding Order of O2 & More The bond dissociation energy of N 2 is very high, i.e. 941kJmol -1. (iii) Oxygen, 02. The bond order in O 2, is 6-2/2= 2, which corresponds to a double bond. This is consistent with the big bond energy of 496kJ mol -1 of oxygen molecule. The filling of molecular orbitals leaves 2 unpaired electrons in each of the π * (2p y) and π * (2p z ...
Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
Molecular Orbital Theory - Chemistry Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . Each oxygen atom contributes six electrons, so the diagram appears as shown in .
Molecular Orbital (MO) Diagram for O2(-) - YouTube When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the othe...
Electronic valence molecular orbital configuration of "O ... You'll need the molecular orbital (MO) diagram of O2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get O2. Two 2s orbitals combine to give a σ2s bonding and σ* 2s antibonding MO.
8 - Drawing Molecular Orbital Diagrams — Flux Science The way these bonds are placed on any molecular orbital diagram is according to how the atomic orbitals that make the MOs mix. In that mixing, there are two factors to consider: (1) atomic symmetry and (2) mixing. Image via Chegg Symmetry As a bond occurs, a bond or internuclear axis - the line that connects the nuclei of two bonded atoms - forms.













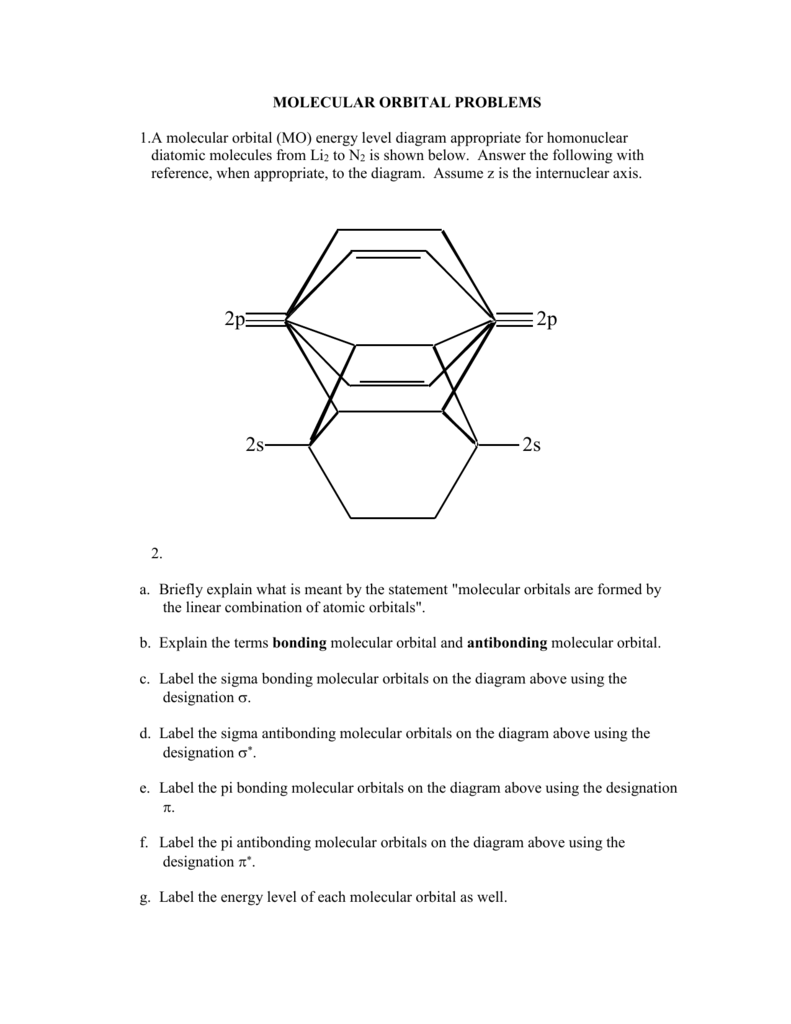





0 Response to "40 o2+ molecular orbital diagram"
Post a Comment