41 what is a lone pair in a lewis diagram
SiS2 Lewis Structure, Molecular Geometry, Hybridization ... It is the presence of lone pairs of electrons that makes the molecule quite active in a higher energy state. These lone pairs of electrons further change the molecular geometry of the molecule from bent shape to linear making the bond angle 180°. Solved Question 5 of 30 > Draw the Lewis structure for ... Lewis structure of BeF2- • valenec electron for all atoms- Be= 2, 2F = 2×7= 14, total electron= 16 electrons • electrons required to comp …. View the full answer. Transcribed image text: Question 5 of 30 > Draw the Lewis structure for BeF2, including lone pairs. What is the molecular shape of BeF,?
techiescientist.com › hno3-lewis-structureHNO3 Lewis Structure, Molecular Geometry, Hybridization, and ... Mar 28, 2022 · Apart from this, the covalent bonds and lone pairs determine the structure of a molecule. The electron pairs participating in a bond repulses each other; the repulsive force is the highest between two lone pairs. It is less in the case of lone pair and bond pair, and the least between bond pairs.

What is a lone pair in a lewis diagram
VSEPR Theory: Introduction - YouTube To see all my Chemistry videos, check outhttp://socratic.org/chemistryThis is an introduction to the basics of VSEPR Theory. VSEPR theory is a set of rules f... What is the molecular shape of HNO? - AskingLot.com Hydrogen Cyanide In this example, HCN, the Lewis diagram shows carbon at the center with no lone electron pairs. The carbon and nitrogen are bonded through a triple bond which counts as "one electron pair". Hence the molecule has two electron pairs and is linear. It boils at 25oC, and thus is a gas a room temperature. PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 lewis structure In this lewis structure of PCl 3, center phosphorus atom has made three single bonds with three chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. Also, there are no charges on atoms in PCl 3 lewis structure. Steps of drawing PCl 3 lewis structure
What is a lone pair in a lewis diagram. SiO2 Lewis Structure| Step By Step Construction - What's ... This molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound. What is the structure of SiO2? Silica is a crystalline polymer with a tetrahedral structure. The silicon atom is attached with four oxygen atoms to form a single bond with each oxygen atom at tetrahedral angles. 41 lewis dot diagram h2o - Wiring Diagram Source Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions. H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. techiescientist.com › h3o-lewis-structureH3O+ Lewis Structure, Geometry, Hybridization, and MO Diagram ... Apr 04, 2022 · The reason for the polarity also emerges due to the presence of lone pair on the oxygen atom in the H3O+ molecule. The net dipole comes out to be some non-zero value that makes H3O+ a polar molecule. H3O+ Molecular Orbital (MO) Diagram. A molecular orbital diagram of any molecule gives us an idea about the mixing of orbitals in the molecule. › definition-of-lewis-structureLewis Structure Definition and Example - ThoughtCo A Lewis structure is a diagram that shows the covalent bonds and lone electron pairs in a molecule. Lewis structures are based on the octet rule. While Lewis structures are useful for describing chemical bonding, they are limited in that they do not account for aromaticity, nor do they accurately describe magnetic behavior. Definition
Drawing Lewis Structures to Determine Molecular Geometry ... Draw Lewis structures AND predict the molecular geometry of the following compounds or polyatomic ions: 1. CCl4 Tally the valence electrons C = 1 × 4 = 4 Cl = 4 × 7 = 28 Total: 32 valence electrons or 16 pair C is in center with 4 Cl’s around—this uses 8 electrons or 4 pair. We still have 12 pair—place 3 pair on each terminal Cl atom. Count and make sure that all atoms … What Is A Lone Pair Of Electrons - questionfun.com What is a lone pair in a Lewis diagram quizlet? Lone Pair. a pair of valence electrons that are not shared with another atom. Only $2.99/month. Single Bond. a bond in which two atoms share one pair of electrons. What is a lone pair in Vsepr? The extra pairs of electrons on the central atom are called ' lone - pairs '. … Lone Pair - Chemistry Definition - ThoughtCo A lone pair is an electron pair in the outermost shell of an atom that is not shared or bonded to another atom. It is also called a non-bonding pair. One way to identify a lone pair is to draw a Lewis structure. The number of lone pair electrons added to the number of bonding electrons equals the number of valence electrons of an atom. Draw the Lewis structure of XeCl_4 showing all lone pairs ... Draw the Lewis structure of CIBr_3 showing all lone pairs. A CIBr_3 molecule is polar. nonpolar. Identify the molecular geometry of CIBr_3. square planar trigonal pyramidal linear trigonal planar see-saw square pyramidal trigonal bipyramidal bent...
en.wikipedia.org › wiki › Lewis_acids_and_basesLewis acids and bases - Wikipedia A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH 3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me 3 B) is a Lewis acid as it is capable of accepting ... NH2OH lewis structure, Molecular geometry, and Bond angle Lone pairs are those represented as dots in the lewis diagram that do not take part in the formation of bonds and are also called nonbonding electrons. So, in the NH2OH lewis structure, there are 4 bond pairs means 8 bonding electrons and 3 lone pairs (1 on central atom + 2 on oxygen atom) are present. Lewis Structure of SO2 (2021 UPDATED) Complete Guide There are many similar Lewis Structures of SO2 depending on the shape, geometry, and lone pairs. The SO2 Lewis Structure of water, hydrogen sulfide, nitrogen dioxide, and ozone has the same shape, which is bent. Hydrogen sulfide, water, sulfur dioxide, and nitrogen dioxide have two sigma bonds. Lastly, Ozone and SO2 have one lone pair on their ... CN- lewis structure, molecular orbital diagram, and, bond ... In this article, we will study the Cyanide (CN-) lewis structure, molecular orbital diagram(MO), its bond order, formal charges, and hybridization. Cyanide can be a colorless gas in the form of hydrogen cyanide, sodium cyanide, potassium cyanide, etc. It is released as a decay product of many plants and it is one of the most poisonous chemicals in chemistry. Some bacteria, fungi, …
What is the Lewis structure of CO? | Socratic This often looks wrong to a student who is used to seeing double bonds on oxygen. Students are typically taught an electron-counting method, which goes as follows: Count the number of valence electrons per atom. Draw out a predicted atom connectivity. Place all electrons in predicted spots. Where there are electron pairs, construct one bond line for each electron pair.
Solved 1. What is a lone pair in a lewis diagram? (1pt) a ... An unshared pair of electrons e. The electrons that are not counted when determining if the octet of an atom has been satisfied. Question: 1. What is a lone pair in a lewis diagram? (1pt) a. A bonding pair of electrons b. A shared pair of electrons 2 c. A molecule with only two electrons d. An unshared pair of electrons e. The electrons that ...
Carbon monoxide (CO) Molecule Lewis Structure Lewis structure of CO molecule contains a triple bond. Both Carbon and Oxygen atoms have one lone pair in their valence shells. CO lewis structure In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. However, oxygen atoms has a +1 charge and carbon atom has a +1 charge.
PBr3 Lewis Structure, Molecular Geometry, Polarity, and ... And, N stands for 1 lone pair of electrons on the central P. So, it is AX3N. Look at the above diagram. We can see that for PBr3 there are four electron-dense areas out of which three are bonded zones and one is lone pair around the central atom. Hence, the required shape is trigonal bipyramidal. The bond angle is around 109.5 degrees approx.
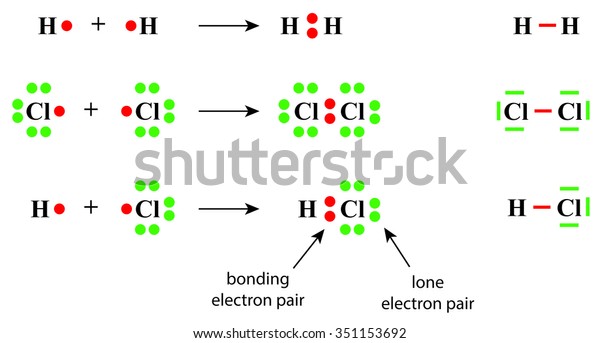
PDF bonding pair lone pair - Westfield State University Bonding pairs and lone pairs: since an orbital can hold two electrons we usually talk about electrons in pairs. A bonding pair is the pair of electrons that are being shared. A lone pair are a pair of electrons that are not being shared. Lewis structures are a way of representing the atoms and electrons which constitute a bond.
I3 Lewis Structure, Molecular Geometry, Hybridization ... Vor 2 Tagen · Forming the single bonds with the other two iodine, we find out that there are 3 lone pairs and 2 bond pairs for the central iodine. 5. Checking the formal charge, we put the negative charge outside as per the diagram above. Thus, lewis structure is done. Hybridization of I3. The hybridization of I3 (Triiodide ion) is sp3d.
What is the NH4 Lewis Structure? - Speeli Lewis structures show the bonding between a molecule's atoms and the lone pairs of electrons, possibility existing in the molecule. Lewis structures are also known as Lewis dot diagrams, Lewis dot structures, Lewis dot formulas, Lewis electron dot structures, or electron dot structures.
What is Lone Pair Effect? - BYJUS A lone pair in chemistry refers to a pair of valence electrons that in a covalent bond are not exchanged with another atom and is often called an unshared pair or non-bonding pair. In the outermost electron shell of atoms, lone pairs are found. By using a Lewis structure, they can be defined.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.
What are lone pairs and how are they represented in a ... What are lone pairs and how are they represented in a Lewis dot diagram? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram 1 Answer anor277 Jun 23, 2018 These are conceived to be pairs of electrons present on the central atom, that DO NOT participate in bonding.... Explanation: And ammonia is a go to example....
Lone pair - Wikipedia Lone pairs (shown as pairs of dots) in the Lewis structure of hydroxide In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms.
CO2 Lewis Structure (2021 UPDATED) All You Need To Know CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula.
NO2+ Molecular Geometry - Science Education and Tutorials 1.Determine the number of lone pairs of electrons in the core nitrogen atom of the NO2+ Lewis structure. Because the lone pairs of electrons on the nitrogen atom are mostly responsible for the NO2+ molecule geometry planar, we need to calculate out how many there are on the central nitrogen atom of the NO2+ Lewis structure.
Lewis Structures - University of Illinois Urbana-Champaign The Lewis dot structures of the individual, non-metal atoms give a good indication of the bonding possibilities for the atoms. Carbon tends to form 4 bonds and have no lone pairs. Nitrogen tends to form three bonds and have on e lone pair. Oxygen tends to form two bonds and have two lone pairs.
Lewis Dot Symbols and Lewis Structures | Boundless Chemistry Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 lewis structure In this lewis structure of PCl 3, center phosphorus atom has made three single bonds with three chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. Also, there are no charges on atoms in PCl 3 lewis structure. Steps of drawing PCl 3 lewis structure
What is the molecular shape of HNO? - AskingLot.com Hydrogen Cyanide In this example, HCN, the Lewis diagram shows carbon at the center with no lone electron pairs. The carbon and nitrogen are bonded through a triple bond which counts as "one electron pair". Hence the molecule has two electron pairs and is linear. It boils at 25oC, and thus is a gas a room temperature.








0 Response to "41 what is a lone pair in a lewis diagram"
Post a Comment