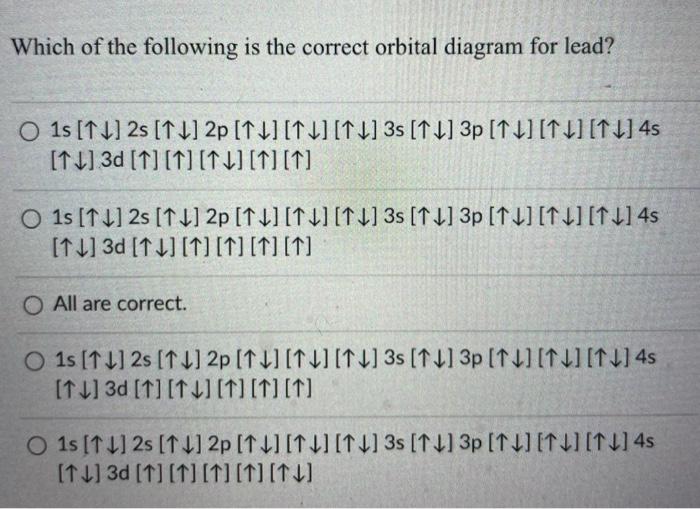
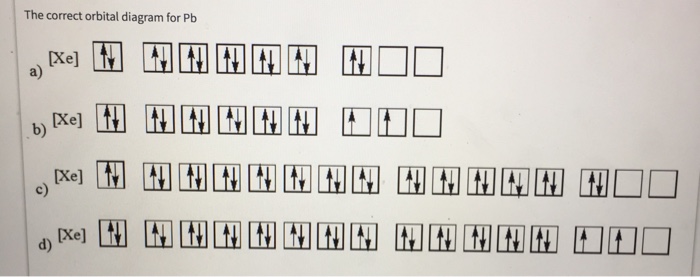
42 orbital diagram for lead
What is the correct configuration for lead? answer choices [Rn] 6s2 5d10 4f14 6p2 [Xe] 6s2 6d10 6p2 [Xe] 6s2 5d10 6p2 [Xe] 6s2 5d10 4f14 6p2. Tags: Question 38 . SURVEY . 30 seconds . Q. This orbital diagram represents: answer choices . C. B. N. O. Tags: Question 39 . SURVEY . 30 seconds . Q. What is incorrect about this orbital diagram? answer ... What is the orbital diagram of the atom Lead Pb 82. close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram of the atom Lead Pb 82. check_circle Expert Answer. ... Q: What is the orbital diagram of the atom phosphorous? A: ...
3:49Either way, the Lead electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 ...2 Jul 2020 · Uploaded by Wayne Breslyn

Orbital diagram for lead
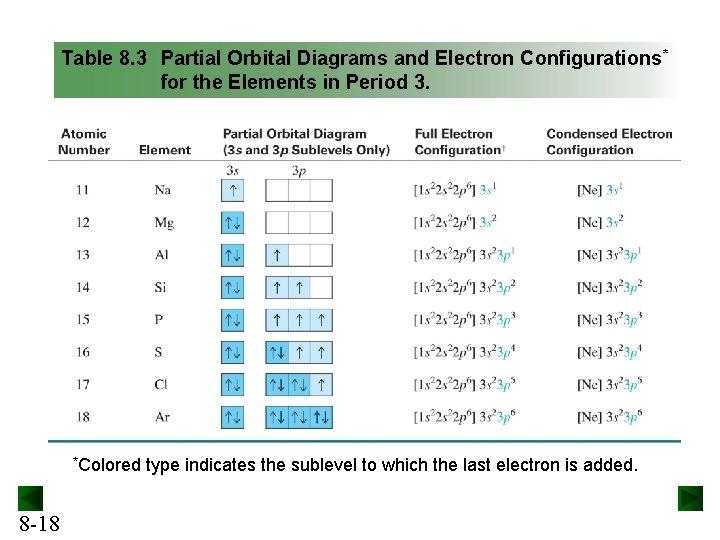
required and write a partial orbital diagram. PROBLEM: Use partial orbital diagrams to describe how mixing of the atomic orbitals of the central atom(s) leads to hybrid orbitals in each of the following: (a) Methanol, CH. 3. OH (b) Sulfur tetrafluoride, SF. 4 (a) CH. 3. OH. The electron- group arrangement is tetrahedral around both the C and ... Answer: This is the orbital diagram of lead: But in science, it’s pretty difficult to work with these diagrams; not to mention that are utterly wrong. Instead, electron configurations are used. Lead (Pb) has an atomic number (Z) of 82. That means it has 82 protons on it’s nucleus. On Pb’s funda... 3 May 2018 — Electron configurations tell you the occupied electron orbitals for any given element. This is important in physics and chemistry because ...
Orbital diagram for lead. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. lead (Pb; Z = 82) PLAN: The atomic number gives the number of electrons, and the periodic table shows the order for filling orbitals. The partial orbital diagram includes all electrons added after the previous noble gas except those in filled inner sublevels. 23.02.2016 · The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: A few things to keep in mind here: The reason we show three p-orbitals is ... Skylab was the first United States space station, launched by NASA, occupied for about 24 weeks between May 1973 and February 1974. It was operated by three separate three-astronaut crews: Skylab 2, Skylab 3, and Skylab 4.Major operations included an orbital workshop, a solar observatory, Earth observation, and hundreds of experiments. ...
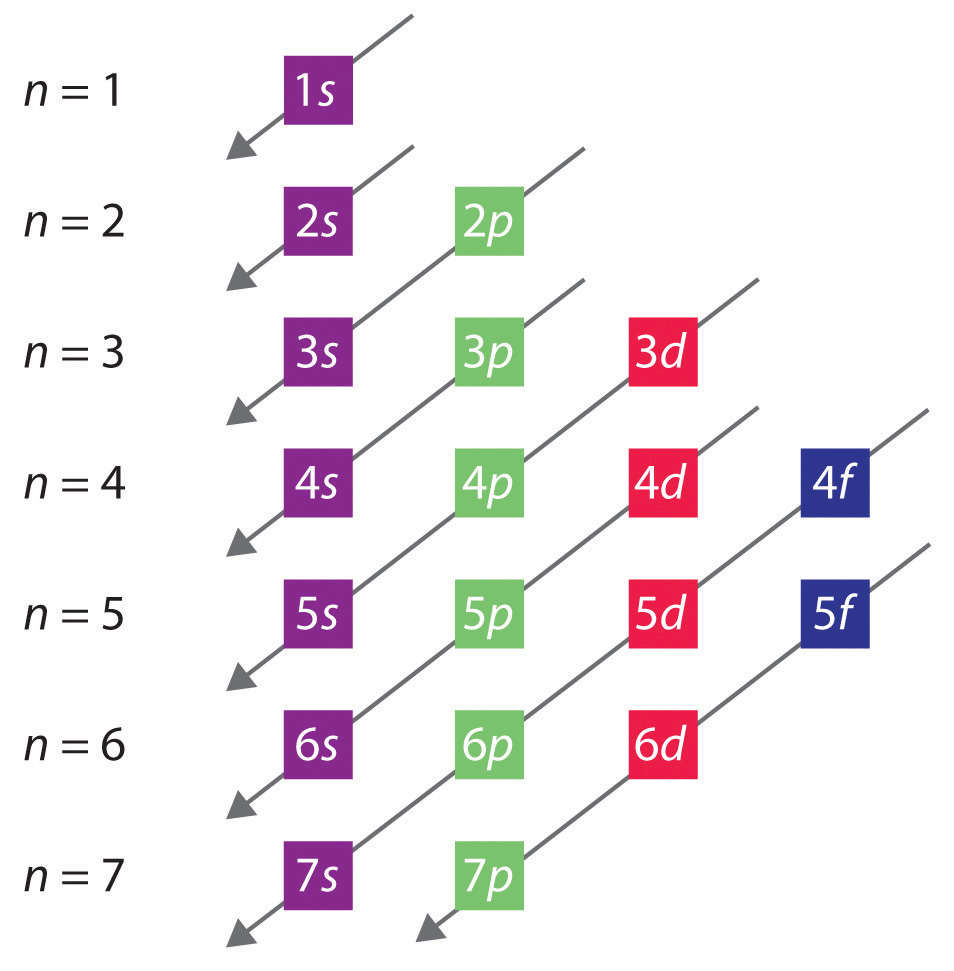
with its neighbors. Strong orbital interactions lead to a more disperse band (green curve) which becomes successively flatter with weaker interactions (blue and red curves). when m= nwhere is simply the energy of an electron in one atomic orbital and Z ˜ m H˜ n= (21) when m= (n 1) where is the interaction energy. Now we can evaluate the ... orbitals have one electron. Then additional electrons enter each orbital until 2 . electrons are in each orbital. Once all orbitals in a sublevel are filled (each with 2 . electrons), the next electron enters the next higher energy sublevel. The Aufbau diagram below illustrates the order of filling orbitals and sublevels. 6:06Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams ...27 Sep 2013 · Uploaded by The Science Classroom In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or ions by the combination of several contributing structures (or forms, also variously known as resonance structures or canonical structures) into a resonance hybrid (or hybrid structure) in valence bond theory.It has particular value for describing delocalized electrons within certain ...
15.02.2021 · Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration. February 15, 2021 by Sneha Leave a Comment. Nitrogen Electron Configuration: When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is science. For those who are new and are not aware of the divisions of the subject, so here we will help you out with that. Oxygen ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... The ground state electron configuration of ground state gaseous neutral lead is [Xe].4f14.5d10.6s2.6p2 and the term symbol is 3P0. 10 Feb 2021 — Lead Electron Configuration: We all have to go to school after a certain age and there we have to deal with many subjects at a time.
Electron Configuration, [Xe] 4f14 5d10 6s2 6p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p2. Orbital Diagram.Atomic Number: 82Period: 6Atomic Weight: 207.2 Isotopes
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
Although we have ignored the remaining p-orbitals, their inclusion in a molecular orbital treatment does not lead to any additional bonding, as may be shown by activating the fluorine correlation diagram below. Another type of MO (the π orbital) may be formed from two p-orbitals by a lateral overlap, as shown in part A of the following diagram. Since bonds consisting of occupied π-orbitals ...
26 Apr 2021 — The purpose of introducing quantum numbers has been to show that similarities in the electron arrangement or electron configuration lead to ...
... Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron configuration, Chemical Properties lead, ...
3 May 2018 — Electron configurations tell you the occupied electron orbitals for any given element. This is important in physics and chemistry because ...
Answer: This is the orbital diagram of lead: But in science, it’s pretty difficult to work with these diagrams; not to mention that are utterly wrong. Instead, electron configurations are used. Lead (Pb) has an atomic number (Z) of 82. That means it has 82 protons on it’s nucleus. On Pb’s funda...
required and write a partial orbital diagram. PROBLEM: Use partial orbital diagrams to describe how mixing of the atomic orbitals of the central atom(s) leads to hybrid orbitals in each of the following: (a) Methanol, CH. 3. OH (b) Sulfur tetrafluoride, SF. 4 (a) CH. 3. OH. The electron- group arrangement is tetrahedral around both the C and ...

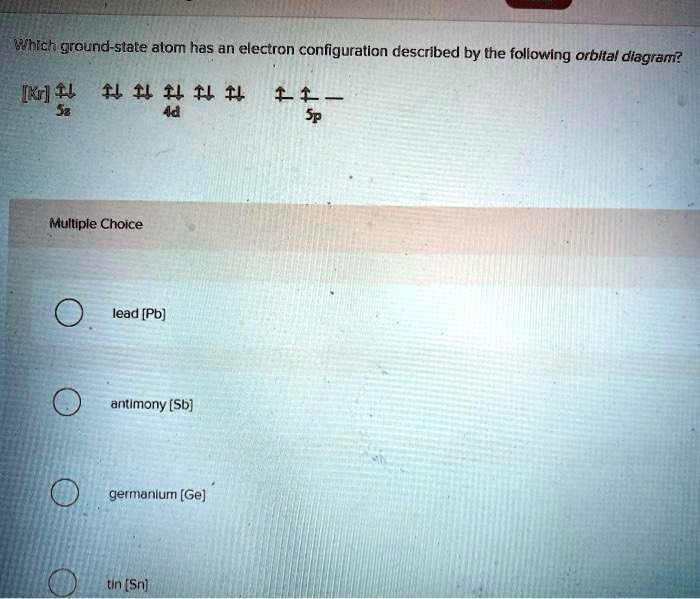
1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2






/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)












0 Response to "42 orbital diagram for lead"
Post a Comment