36 electron dot diagram for aluminum
16/12/2021 · BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature. It can exist in both monomeric and 1-D polymeric forms. The properties of beryllium chloride are similar to aluminum chloride owing to the diagonal relationship of beryllium with aluminum.
Aug 18, 2021 — And what is the Lewis structure of Al? ... Answer: Aluminum belongs to group IIIA of the periodic table and therefore has three valence electrons.
Dec 19, 2019 · An atom of Neon in the gas phase, for example, gives off energy when it gains an electron to form an ion of Neon. a. Define the term ion and explain how the electron dot structure of a s- or p-block The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. electron configuration …

Electron dot diagram for aluminum
Problem. What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: . The valence electron configuration for selenium is 4s 2 4p 4.In the highest-numbered shell, the n = …
What is the Lewis dot structure of aluminum? The Lewis dot structure for aluminum includes the symbol, "Al," and a total of three dots around the symbol. What is the Lewis structure of beryllium? Since beryllium only has two valence electrons, it does not typically attain an octet through sharing of electrons.
Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH3 Lewis structure there are a total of 8 valence electrons available. What is the Lewis dot structure for aluminum? The Lewis dot structure for aluminum includes the symbol, "Al," and a total of three dots around the symbol.
Electron dot diagram for aluminum.
aluminum ion lewis dot structure. Aluminum has three 3 valence electrons, which can be given off or shared to produce the aluminum ion, Al{eq}^{3+} electron dot structure of carbonate ion, Toothpastes containing sodium hydrogen carbonate sodium bicarbonate and hydrogen peroxide are widely used, Step-1 : To draw the lewis Dot structure of CO2, we have to find out the valence electrons of carbon ...
Lewis structure is also known as electron dot structure or Lewis dot structure because the valence electrons are represented as dots in the Lewis structure of the molecule. It is the two-dimensional structure in which every atom in the molecule tends to complete its octet either by sharing or gaining or losing electrons.
Aluminum is known to contain 3 valence electrons. As such, we must draw a total of three dots around the chemical symbol... See full answer below.1 answer · Top answer: The given statement is true Aluminum is known to contain 3 valence electrons. As such, we must draw a total of three dots around the chemical symbol...
Hydrogen electron configuration is 1s 1.Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.. The first element of the periodic table is hydrogen and its position at the beginning of the periodic table.
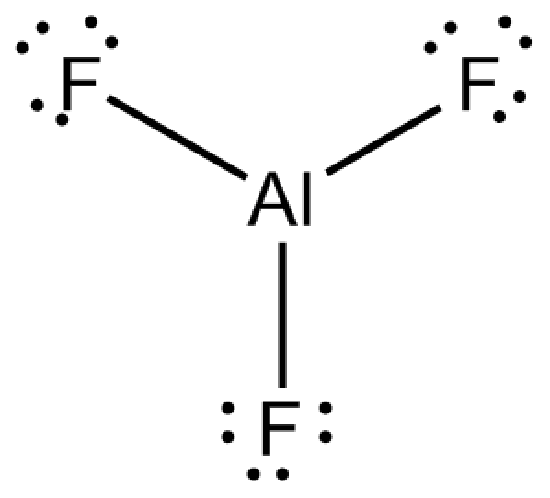
Since aluminum is the least electronegative atom, it must be placed in the center, and fluorine only forms one bond. Considering this, we have the Lewis structure of AlF 3 (upper image). Shared electrons are highlighted with green dots to distinguish them from unshared ones.
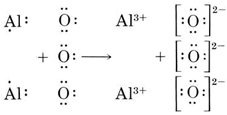
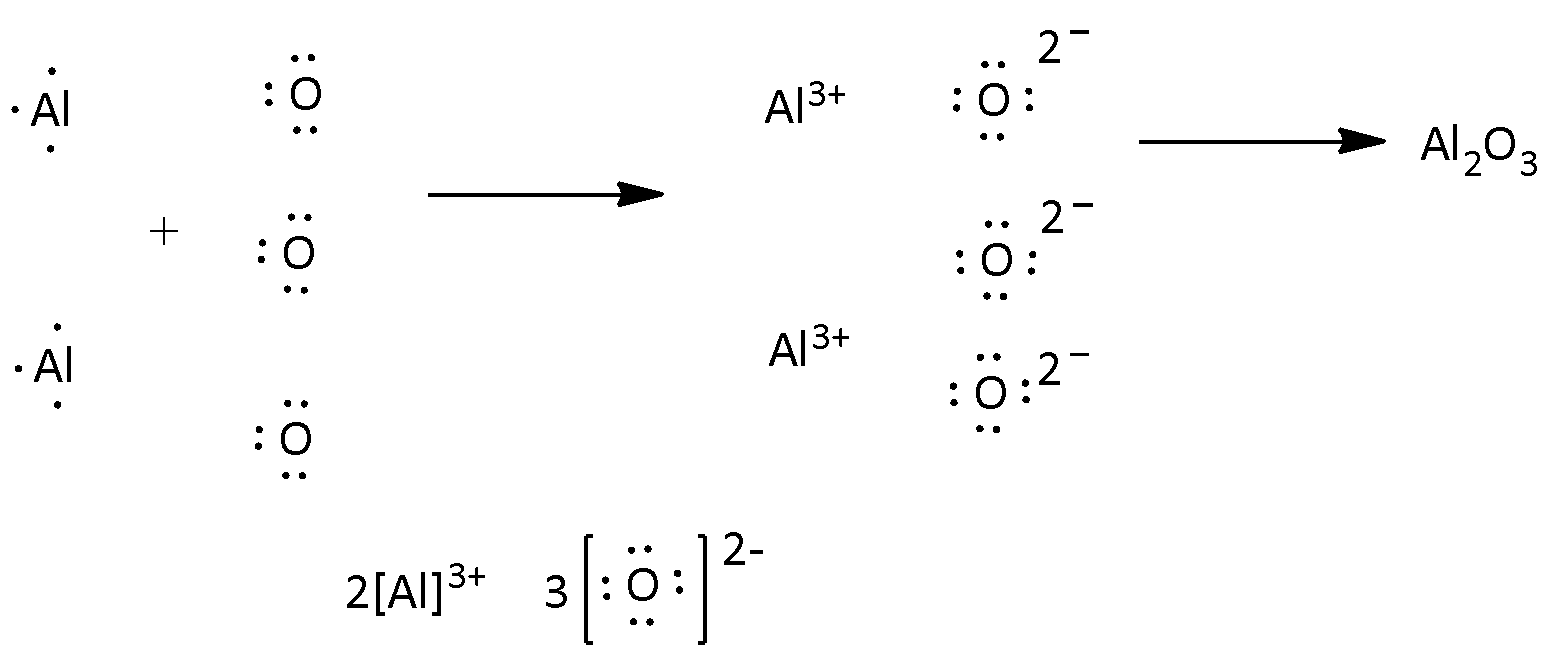
No Comments on Lewis Structure of Al2O3, Aluminum Oxide Aluminium oxide is a solid ionic compound, made from atoms of one metal (Aluminum) that have lost three electrons each to become +3 cations, and atoms of a non-metal (oxygen) which have gained two electrons each to become -2 anions.
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 …
What do the dots represent in the Lewis dot structure for aluminum? — What do the dots represent in the Lewis dot structure for aluminum? What is the ...
A Lewis electron dot diagram ... The valence electron configuration for aluminum is 3s 2 3p 1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: The valence electron configuration for selenium is 4s 2 4p 4.
Step 3: Lewis Structure is also known as an electron-dot structure since we use dot notations to represent the valence electrons surrounding the atoms. Let us have a look at the schematic sketch of HF after we have placed the dot electrons: Step 4: Now, we will check the octet rule.
The Lewis structure depicts the best possible placement or arrangement of electrons. Aluminum Oxide is an ionic compound i.e. the bonds between aluminum and oxygen atoms are formed through the transfer of electrons from one atom to another atom. The Lewis structure for Aluminium Oxide (Al2O3) is represented as:
What is the Lewis dot structure for MG? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
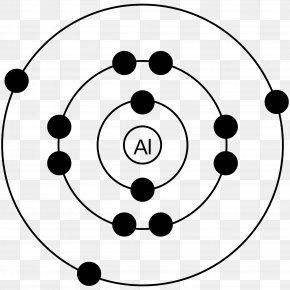
Diagram of the nuclear composition and electron configuration of an atom of aluminium-27 (atomic number: 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (blue). 13 electron s (green) bind to the nucleus, successively occupying available electron shells (rings). Selenium Sulfide is an orange-yellow powder that is soluble in carbon disulfide ...
Example 1 · The valence electron configuration for aluminum is 3s 23p 1. So it would have three dots around the symbol for aluminum, two of them paired to ...
A Lewis structure is a representation of atoms of elements using dots. These dots show the number of outermost electrons in the atom. These outermost electrons are involved during ionic bonding. We can see from the image attached that in ionic bonding, electrons are transferred from the metal to the non metal to form the ionic compound.
Electron Configuration Chart of All Elements (Full Chart) June 10, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
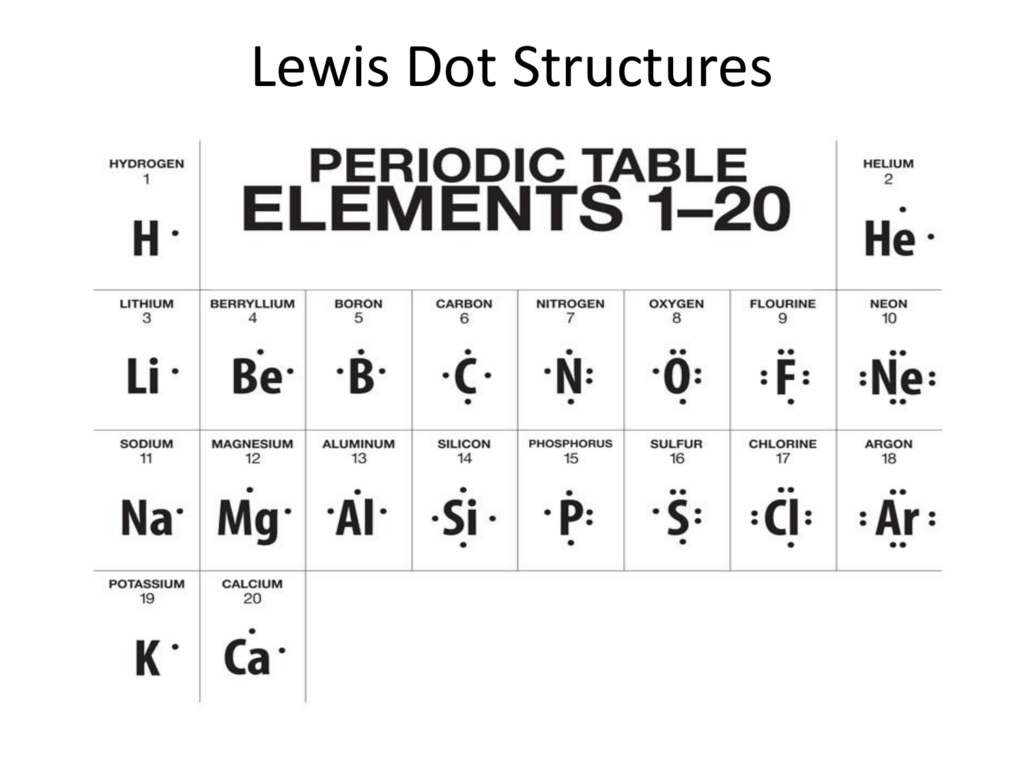
2. Draw diagrams: Create an electron dot diagram for each of the elements below. Use the Gizmo to you do this. To check your work, turn on Show electron dot diagram. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
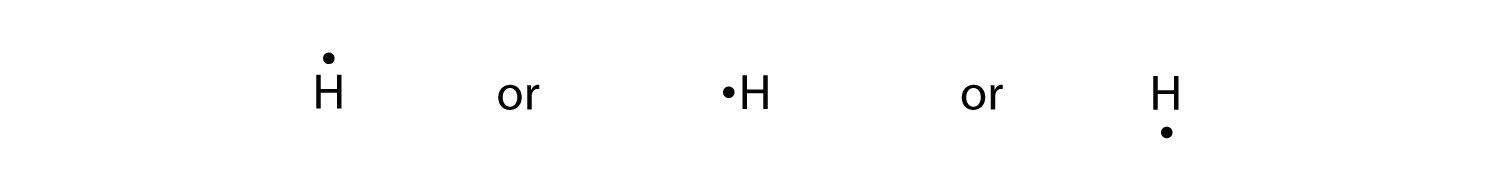
The Lewis structure of a compound also indicates the presence or absence of lone pairs of electrons which provides information on the compounds chemical reactivity and physical properties. Lewis Electron Dot Diagrams Introduction Lewis electron dot diagrams Diagram Drawing Hydrogen The Lewis electron dot diagram for hydrogen is simply.
03/12/2021 · Dot Diagram Practice for Covalent Compounds (multiple copies) Ch. 7 Test Review - optional assignment ; LABS. Electron Dot Diagram Lab for Covalent Compounds with covalent data table and example answer to data table entry 1; Molecular Model Building Lab with data table - (three periods) Molecular Model Building Lab with shorter data table (two ...
The electron dot diagram which is also known as the Lewis electron dot diagram is a diagram that shows an atom's valence electrons by placing dots, which represent the valence electrons of the element, around the elements symbol. The element for which the electron dot diagram is found = Neon, Ne. The electronic configuration of neon ...
Read also electron and learn more manual guide in lewis structure electron dot structure for hoi A lewis structure also called lewis dot formulas lewis dot structures or electron dot structures are pictorial diagrams that represent the bonding between atoms in a compound and the placement of electrons. Lewis electron dot diagram for SiF4.
Electronic Configuration For Aluminium Al Spdf Trick Chemistry Atomic Number 13 Youtube. Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes. 11 Na Sodium Electron Shell Structure Schoolmykids Matter Worksheets Element ...
CHAPTER 7. A (n) _______ bond is a chemical bond that results from sharing a pair of electrons between two atoms. A (n) ______ results from a transfer of one or more electrons from one atom or molecule to another. Nice work! You just studied 91 terms! Now up your study game with Learn mode.
Aluminum has three valence electrons, oxygen has six valence electrons. The formula of aluminum oxide isAl2O3 ...1 answer · Top answer: Hint: We can say that electron dot structure is nothing but a representation of Lewis dot diagram of the valence electrons of an atom that uses dots around ...
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
Q. Draw the lewis structure for aluminum sulfide ionic compound and what chemical formula does the lewis theory predicts? Solved • Sep 6, 2021 Lewis Dot Structures: Ions Q. Draw a Lewis structure for ClO4- in which the central Cl atom obeys the octet rule. How many are the unshared pairs (lone pairs) on the central Cl ...
Correct answers: 3 question: Write the electron configuration for each element, then show with an electron dot diagram which elements lose and gains and electrons and finally make sure to include the correct chemical formula for each pair. 1. Sodium + fluorine 2. Calcium + oxygen 3. Calcium + fluorine 4. Lithium + oxygen 5. Magnesium + nitrogen 6. Barium + bromine 7. Lithium + nitrogen 8 ...
Electron Dot Diagram For Oxygen. Collected from the entire web and summarized to include only the most important parts of it. Can be used as content for research and analysis. ... Species with incomplete octets are pretty rare and generally are only found in some beryllium, aluminum, and boron compounds including boron hydrides. Let's take a ...
Logo - Lewis Structure Nitrite Sodium Nitride Covalent Bond Nitrate - Smile - Trigonal Planar Molecular Geometry Free PNG is a 1655x1213 PNG image with a transparent background. [3], Sodium nitride can be of reddish brown or dark blue color depending on the synthesis of the compound due to intrinsic properties.
Aluminum has three electrons in its outer shell, and chlorine has seven in its outer shell. Aluminum wants to get rid of its three electrons. This will make the next inner electron shell its outer...
Aluminum 13 3s2 p1 Al 3. I am looking at drawing the lewis structure for the S2- ion. The Lewis structure of S2- is represented by the capital letter S that is surrounded by eight dots including a 2- superscript that indicates the charge of the ion. Correct Part B Draw the Lewis dot structure for.
Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur ...
Mar 24, 2021 — What is the Lewis electron dot diagram for each element? aluminum; selenium. Solution. The valence electron configuration for aluminum is 3s23p1 ...
Answer: Aluminum belongs to group IIIA of the periodic table and therefore has three valence electrons. The symbol of aluminum is Al, which is surrounded by three dots. 2. Do you also know how to find the Lewis symbol? A Lewis symbol is constructed by placing dots representing electrons in external energy around the element symbol.
The aluminum atom loses its three valence electrons. The Mg 2+ ion, the Al 3+ ion, the Na + ion, and the Ne atom are all isoelectronic. For representative elements under typical conditions, three electrons is the maximum number that will be lost. We can also show the loss of valence electron(s) with an electron dot diagram.
Example \(\PageIndex{1}\): What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: \[\dot{Al:} \nonumber\nonumber \]

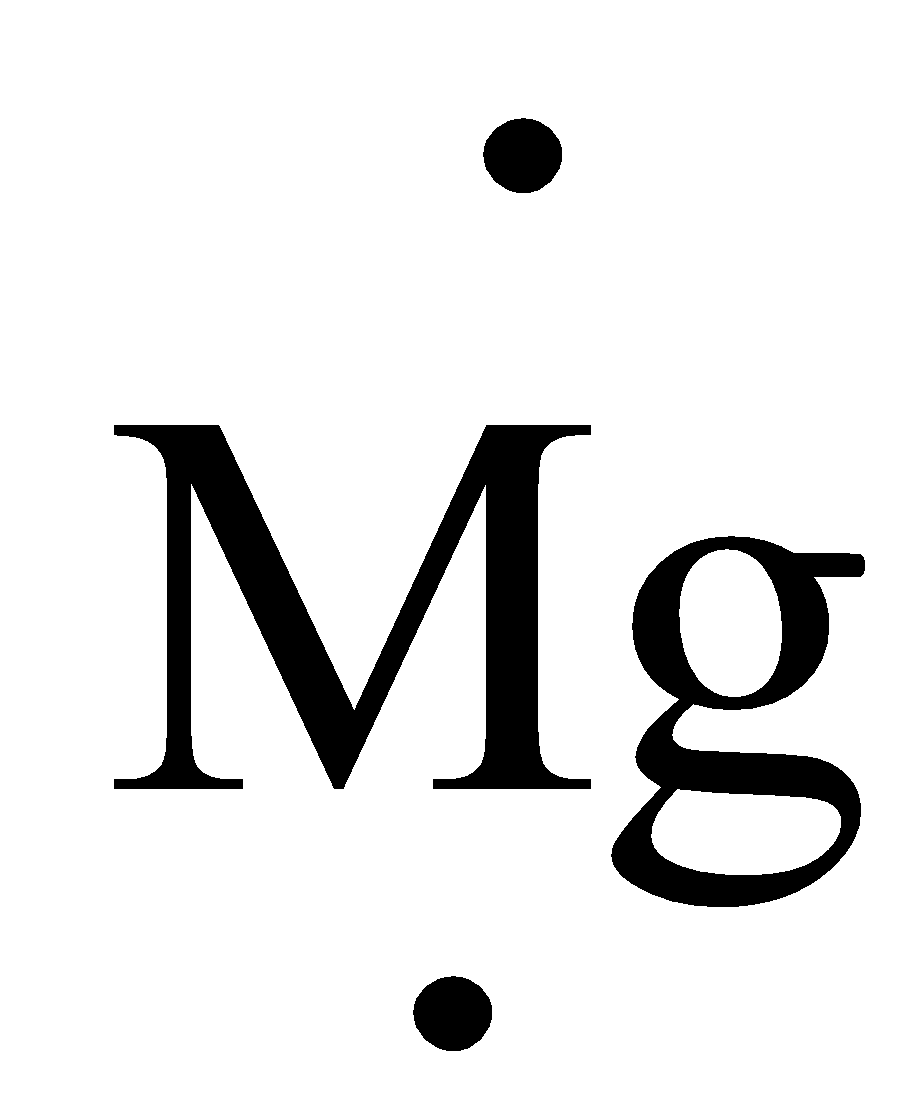
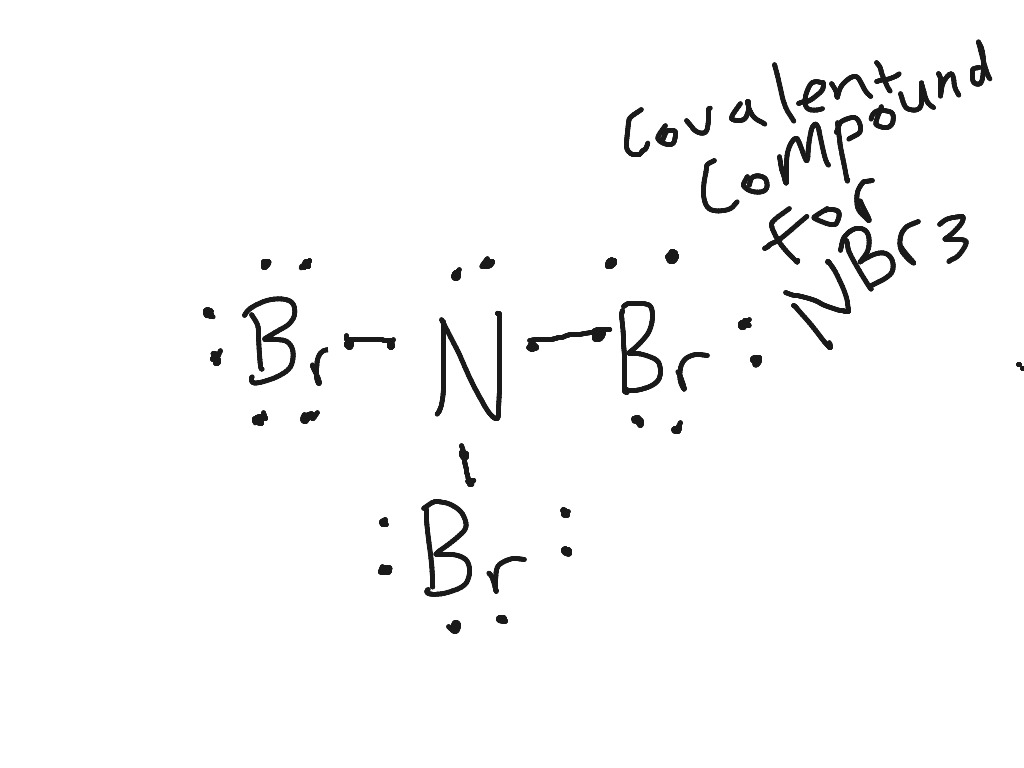










.png)






0 Response to "36 electron dot diagram for aluminum"
Post a Comment