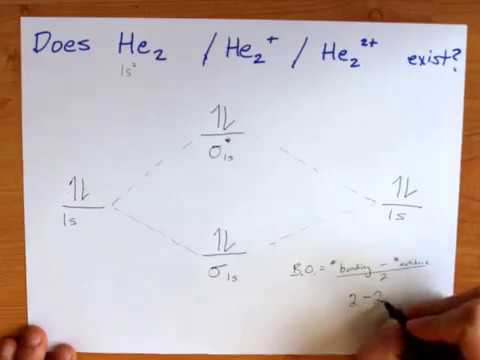
38 which electrons in this diagram contribute to the stability of the he2+ ion?
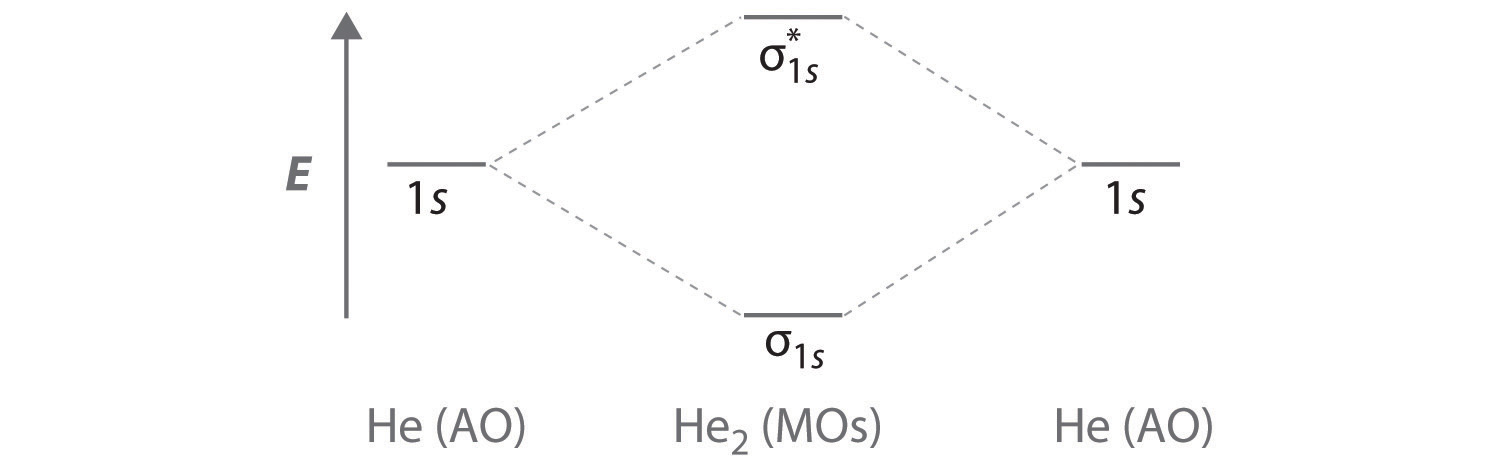
Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding orbitals, the compound is stable. Eg: He + He; same mixing as above. Four electrons, two in the sigma, two in the sigma*. Since there are as many bonding electrons as as antibonding, there is no net bond. He2 is not possible.
Complete Solutions Manual General Chemistry Ninth Edition ... - ID:5dcdb97adce08. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION Ebbing/Gammon. Uploaded by. Sofia Uribe Sanchez. connect to do...
This photo about: Which Electrons In This Diagram Contribute to the Stability Of the He2 Ion?, entitled as Frontiers Which Electrons In This Diagram Contribute To The Stability Of The He2 Ion? - also describes Frontiers and labeled as: ], with resolution 1973px x 1623px
Which electrons in this diagram contribute to the stability of the he2+ ion?
Read Bansal classes chemistry study material for iit jee by S.Dharmaraj on Issuu and browse thousands of other publications on our platform. Start...
the core orbital of an atom make no contribution to the stability of the molecules that ... more highly charged metal ion to the electrons of the ligands.
A dihydrogen molecule contains two bonding electrons and no antibonding electrons so we have. bond order in H2 = (2−0) 2 = 1 bond order in H 2 = ( 2 − 0) 2 = 1. Because the bond order for the H-H bond is equal to 1, the bond is a single bond. A helium atom has two electrons, both of which are in its 1 s orbital.
Which electrons in this diagram contribute to the stability of the he2+ ion?.
Which Electrons In This Diagram Contribute to the Stability Of the He2 Ion? solved which electrons in this diagram contribute to the answer to which electrons in this diagram contribute to the stability of the ion a e electron in the sigma 1s mo and another o which of the following are predicted by the molecular o2 2 f2 2 h2 ‾ he2 ⌂home which of the following are predicted by the molecular ...
The he 2 ion has a total of three electrons. So for h2 the two atoms share the two electrons in a covalent bond which gives it some stability. One electron in the sigma 1s mo and another one in the sigma 1s mo. Two are placed in the bonding orbital the third in the antibonding orbital.
For the molecule He2: a) Draw the molecular orbital diagram. b) Calculate the bond order. c) Would this molecule exist? d) Write the electron configuration o...
Which electrons in this diagram contribute to the stability of the he2 ion. Please note the diagram is for he2 but the he h is very similar eg. After that this he2 molecule will decompose to ground state he atoms. He h forms a very weak bond. The two electrons in the sigma 1s mo.
Molecular ion (He2)+ has a bond order of 0.5 , while (H2)+ has a bond order 0.5. Antibonding orbital is destabilized more in energy than bonding orbital ...5 answers · 27 votes: The question is not that clear, but due to the fact that[math] H^{2+}[/math] doesn't exist ...
Answer (1 of 5): In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4. Molecular Orbitals thus formed are:€1s2€*1s2 It means 2 electrons are in bonding molecular orbitals and 2 are in antibonding molecul...
Which electrons in this diagram contribute to the stability of the he2 ion entitled as molecules free full text which electrons in this diagram contribute to the stability of the he2 ion. He h forms a very weak bond. When an atom loses electrons the ion that is formed has a positive charge. One more electron in bonding than antibonding. One ...
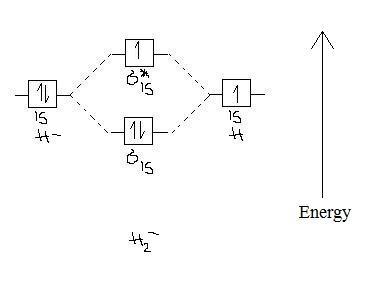
Which electrons in this diagram contribute to the stability of the ion?a. One electron in the sigma 1s MO and another one in the sigma* 1s MO.b. The two electrons in the sigma 1s MO.c. All electrons.d. The electron in the sigma* 1s MO.Energy-level diagram for theHe2+ ion:
Problem: Energy-level diagram for the He2+ ion.Which electrons in this diagram contribute to the stability of the He2+ ion? FREE Expert Solution Show answer Answer: 1/2. 86% (85 ratings) Sign up for free to keep watching this solution Sign up for free. 577,039. students enrolled ...
Construct the molecular orbital diagram for H 2- and then identify the bond order. Next. Practice Problems. What is the bond order of He2+? a. 0 b. ½ c. 1.Nov 02, · For the ion H a) Draw the molecular orbital diagram. b) Calculate the bond order. c) Would this ion exist? d) Write the electron configuration of the ion.
Q. Energy-level diagram for the He2+ ion.Which electrons in this diagram contribute to the stability of the He2+ ion? Q. Label the bonding and antibonding molecular orbitals that result from linear combinations of the 2px atomic orbitals in a homonuclear diatomic...
Transcribed Image Textfrom this Question. Complete the energy-level diagram for H2 Drag the appropriate labels to their respective targets Reset Help σ1s 1s 1s 14 1s H2.
Restricting to pure substances (C=1) and rearranging for phases gives P=3–F. Areas in the phase diagram have two degrees of freedom; one can vary pressure and temperature independently (within limits) and stay within the area. Thus, F = 2 and P = 1 in areas. Lines have one degree of freedom; one can vary pressure or temperature, but to stay on the line the value of the other is …
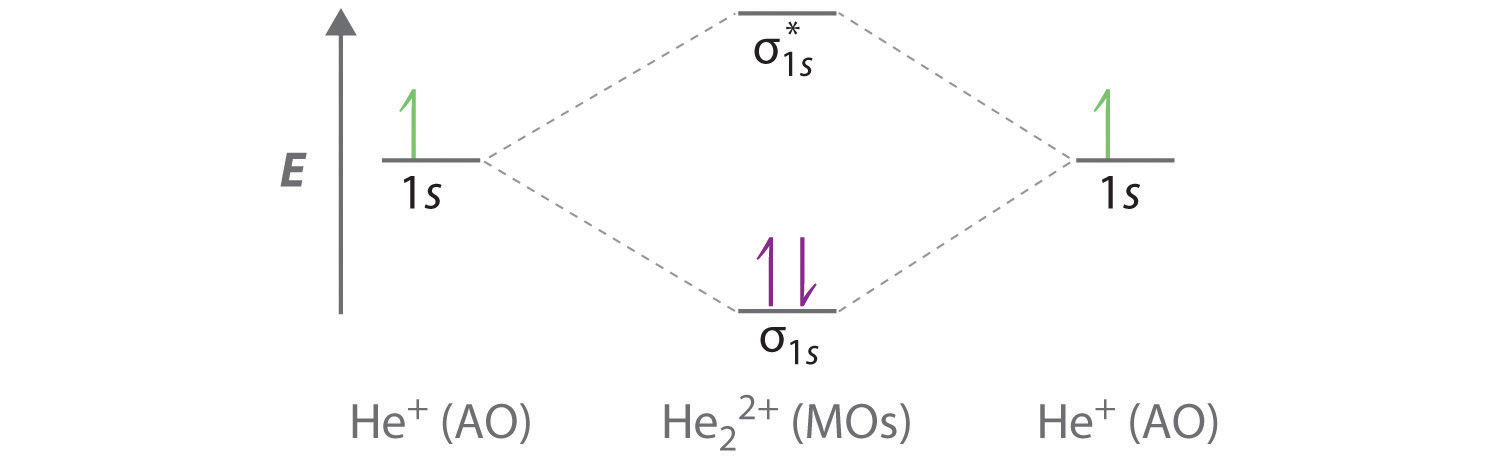
The He2+2 ion has only two valence electrons (two from each He atoms minus two for the +2 charge). We can now fill the molecular orbital diagram.1 answer · Top answer: The He2^+2 ion has only two valence electrons (two from each He atoms minus two for the + 2 charge). We can now fill the molecular orbital diagram.The ...
This photo about: Which Electrons In This Diagram Contribute to the Stability Of the He2 Ion?, entitled as Noble Gas Configuration Video Which Electrons In This Diagram Contribute To The Stability Of The He2 Ion? - also describes Noble gas configuration video and labeled as: ], with resolution ...
OMO has electronics components search system to query: he2, kenmore he2 plus, which electrons in this diagram contribute to the stability of the he2+ ion?, what is the bond order of he2+, h2 lewis structure, molecular orbitals, google, google scholar stock and price.
Bond order= 2 (No. of electrons in bonding molecular orbital)-(No. of electrons in anti-bonding Molecular orbital) = 2 2 − 2 = 0 ∴ H e 2 bond order is 0 .
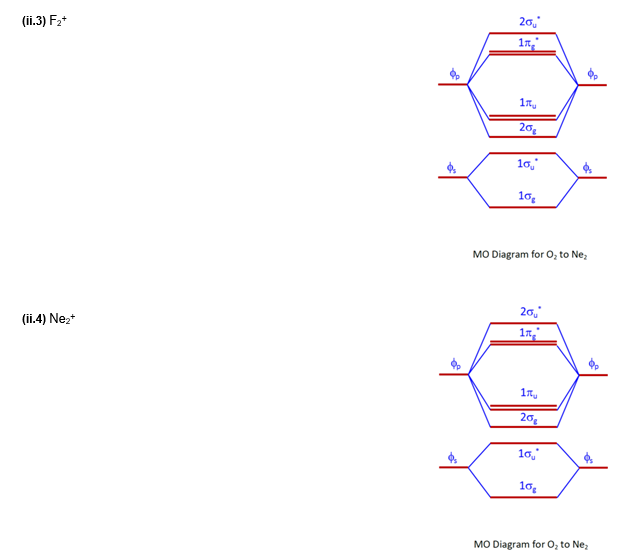
5.10 a. OF- has 14 valence electrons, four in the π 2p* orbitals (see the diagram in the answer to Problem 5.9). b. The net result is a single bond between two very electronegative atoms, and no unpaired electrons. c. The concentration of electrons in the π* orbital is more on the O, so combination with
The electronic configuration of Beryllium is 1s2 2s2. From the electronic configuration it is clear that there is no singly filled atomic orbital present in beryllium. Without the half filled orbital,the overlapping is not possible ,therefore Be2 molecule does not exist.
The first compound in the diagram has two blue and three red atoms. The second compound has two blue and ... An anion is a negatively charged ion with one or more electrons than its neutral atom. A polyatomic ion is made up of more than one atom; the whole unit is the ion. 3.36. Titanium lost four electrons to form Ti4+; it has 22 protons and 18 electrons. 3.37. Negative. …
Which electrons in this diagram contribute to the stability of the he2 ion. Two are placed in the bonding orbital and the third in the antibonding orbital. The other molecular orbital produced s h h shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint between the nuclei where there is a nodal plane. This ion has three electrons. So for h2 the two ...
Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics
You are watching: Which electrons in this diagram contribute to the stability of the he2+ ion? We deserve to use energy-level diagrams to explain the bonding in various other pairs of atoms and also ions where n = 1, such as the H2+ ion, the He2+ ion, and the He2 molecule. Again, us fill the lowest-energy molecule orbitals first while being ...
The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .
The method is illustrated by the flow diagram in Figure 1-1. Scientists may develop a pattern of thinking about their field, known as a paradigm. Some paradigms may be successful at first but then become less so. When that happens, a new paradigm may be needed or, as is sometimes said, a paradigm shift occurs. In a way, the method of inquiry that we call the scientific method …
Dihelium has 2 electrons in a bonding sigma orbital and one in the antibonding sigma. Dihydrogen has one electron in the bonding orbital. Source Molecul Continue Reading Shaz Ansari , studied at Happy Home School Answered 4 years ago H2 is more stable than He2.
Atoms form ions to increase their stability. For example consider sodium which has a single electron in its outermost shell. If it looses this electron it will have a complete octet in its ...
Popular Questions of Class 11 Chemistry. Q:-The mass of an electron is 9.1 × 10 -31 kg. If its K.E. is 3.0 × 10 -25 J, calculate its wavelength.. Q:-Calculate the amount of carbon dioxide that could be produced when
1 answer to which electrons in this diagram contribute to the stability of the ion. Two are placed in the bonding orbital the third in the antibonding orbital. The two electrons in the sigma 1s mo. H2 also has two protons in two nuclei that affect the bonding by contributing two centers of positive charge.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Theodore L. Brown, H. Eugene LeMay Jr., Bruce E. Bursten · 2013 · ScienceFIGURE IT OUT SAMPLE EXERCISE 9.6 Bond order Which electrons in this diagram contribute to the stability of the What is the bond order of the ion?
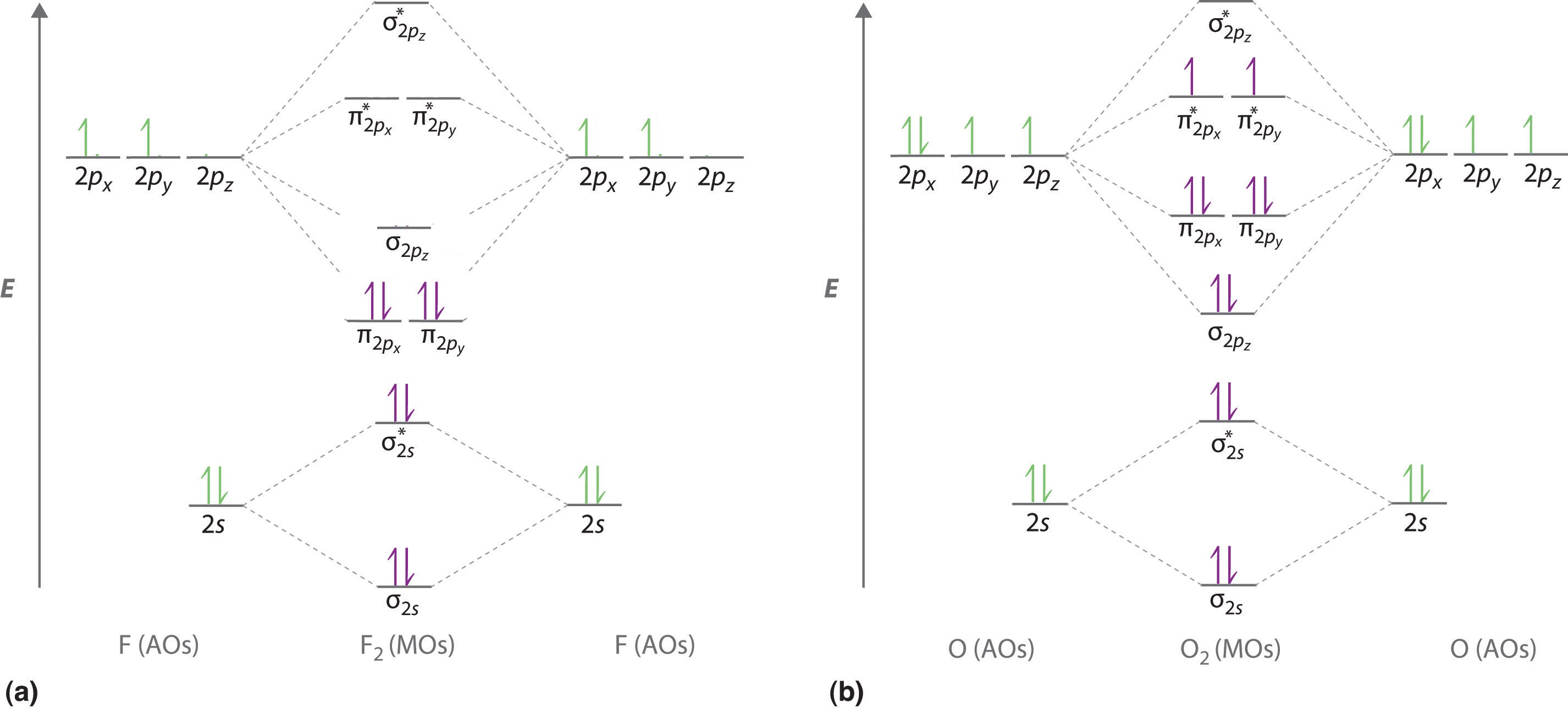
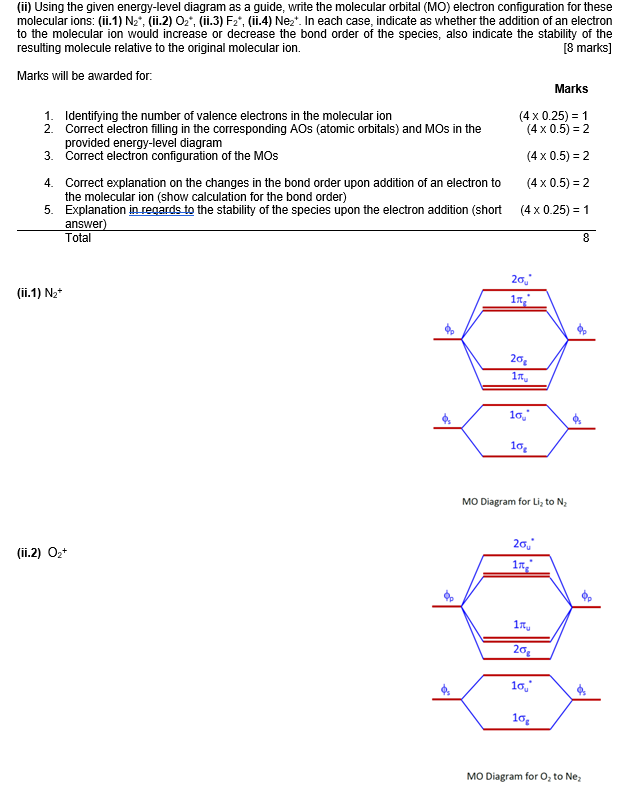
Now consider the structure of N 2.There are 2 × 5 = 10 valence electrons to accommodate. These electrons occupy the five lowest-energy MOs and hence result in the configuration 1σ 2 2σ 2 1π 4 3σ 2.Note that only the orbitals in the lower portion of the diagram of Figure 14 are occupied. This configuration accounts for the considerable strength of the bonding in N 2 and consequently its ...
review from Google Play · Whether you're stumped on geometry or SAT practice, there’s no question too big or too small for Brainly
The molecular orbital diagram is given below: He 2 + contains 3 electrons. Out of these 3 electrons, 2 are present in the σ level and the remaining one is present in the σ* 1s level. Bond order = ½ (N b -N a) = ½ (2-1) = ½ . Since the bond order is half the molecular ion exists but possesses low stability. 2.
22.06.2021 · The mixture of electrons and ions in the acceleration zone means that the thruster does not have the thrust density limitation associated with ion thrusters, although other lifetime considerations limit the achievable thrust densities. As with ion thrusters, M&s tools for Hall thrusters are well advanced and will support scaling to 100 kWe thrusters, although ground …
1 Answer to Which electrons in this diagram contribute to the stability of the ion? a. One electron in the sigma 1s MO and another one in the sigma* 1s MO. b. The two electrons in the sigma 1s MO. c. All electrons. d. The electron in the sigma* 1s MO. Energy-level diagram for theHe2+ ion:
Academia.edu is a platform for academics to share research papers.
Thus we would expect a diatomic molecule or ion containing seven electrons (such as Be + 2 Be 2 +) would have the molecular electron configuration (σ 1 s) 2 (σ ∗ 1 s) 2 (σ 2 s) 2 (σ ∗ 2 s) 1. (σ 1 s) 2 (σ 1 s ∗) 2 (σ 2 s) 2 (σ 2 s ∗) 1. It is common to omit the core electrons from molecular orbital diagrams and configurations ...
Solve The energy-level diagram for the He 2+ ion is shown in FIGURE 9.34. This ion has three electrons. Two are placed in the bonding orbital and the third in the antibonding orbital. Thus, the bond order is Because the bond order is greater than 0, we predict the He 2+ ion to be stable relative to the separated He and He +.
1 answerIn the molecular di-cation of helium ion, two electrons are less than helium atom. Two electrons are to be filled in the molecular orbitals. These two...
Answer (1 of 5): In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4. Molecular Orbitals thus formed are:€1s2€*1s2 It means 2 electrons are in bonding molecular orbitals and 2 are in antibonding molecul...
The two electrons in the sigma 1s MO.c. All electrons.d. The electron in the sigma* 1s MO.Energy-level diagram for theHe2+ ion: Question: Which electrons in this diagram contribute to the stability of the ion?a. One electron in the sigma 1s MO and another one in the sigma* 1s MO.b. The two electrons in the sigma 1s MO.c. All electrons.d.
Which electrons in this diagram contribute to the stability of the he2 ion. If the bond order is greater than 0 we expect a bond to exist and the ion is stable. Energy level diagram for the he 2 ion. The electron in the sigma 1s mo. The valence electrons of he are in the 1s orbital and the 1s orbitals combine to give an mo diagram like that for h 2 or he 2 figure 933. One electron in the sigma ...
So for h2 the two atoms share the two electrons in a covalent bond which gives it some stability. Two are placed in the bonding orbital the third in the antibonding orbital. Energy level diagram for the he2 ionwhich electrons in this diagram contribute to the stability of the he2 ion. The two electrons in the sigma 1s mo.
Re: O3 Lewis Structure O3 has 18 valence electrons (3 * 6e- = 18e-). Because of this, you can't have 2 pairs of double bonds, since it'd give you the incorrect number of electrons. How many triple bonds are in O3? Ozone, or O3 , has two major resonance structures that contribute equally to the overall hybrid structure of the molecule.
Get the detailed answer: Which electrons in this diagram contribute to the stability of theion?a. One electron in the sigma 1s MO and another one in the si

Energy-level diagram for the he2+ ion.which electrons in this diagram contribute to the stability of the he2+ ion?
Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
Draw this out using an energy level diagram: 2 He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular ...
Lewis Electron Dot Structure for O 2 molecule. An oxygen atom has 6 valence electrons in the valence shell and so it needs 2 more to complete the octet. So both the atoms contribute two atoms each for the bond. Hence a double bond is formed. Lewis Electron Dot Structure for the molecule: CO 2. An oxygen atom has 6 valence electrons and a carbon ...






















0 Response to "38 which electrons in this diagram contribute to the stability of the he2+ ion?"
Post a Comment