40 exothermic potential energy diagram
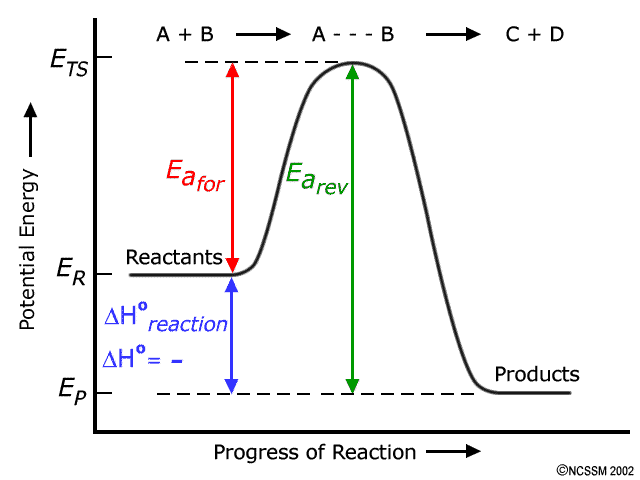
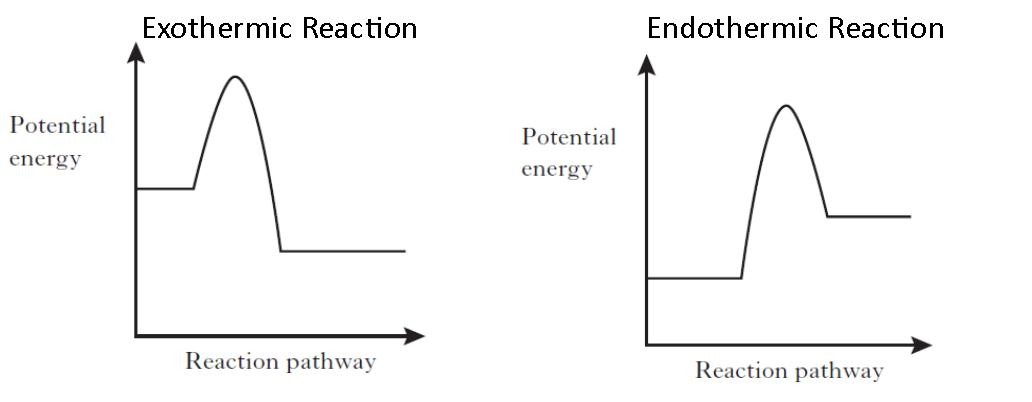
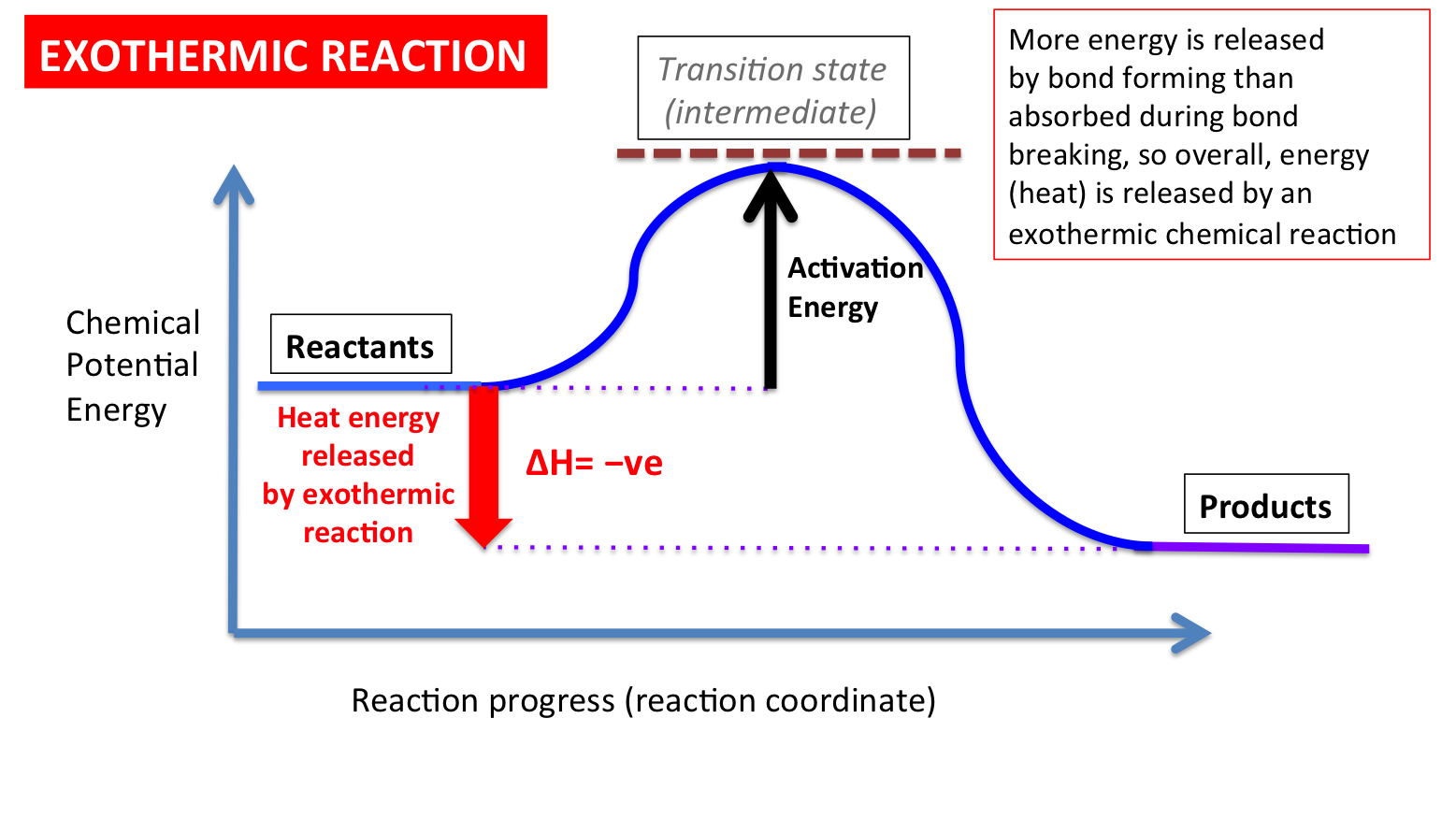
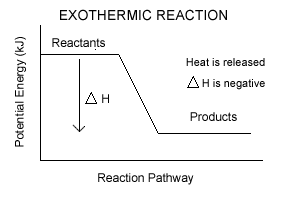
The cumulative energy that is stored in the device is internal energy. It is the amount held by the mechanism of potential and kinetic energy. Enthalpy is specified as the amount of the system’s internal energy plus the combination of the system’s gas pressure and its length. Image of a graph showing potential energy in relation to the process of a chemical reaction. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other words, the products are more stable than the reactants.
In other words, Gibbs free energy is usable energy or energy that is available to do work. Calculating ∆G The standard free energy change of a chemical reaction is expressed as an amount of energy per mole of the reaction product (either in kilojoules or kilocalories, kJ/mol or kcal/mol; 1 kJ = 0.239 kcal) under standard pH, temperature, and pressure conditions.

Exothermic potential energy diagram
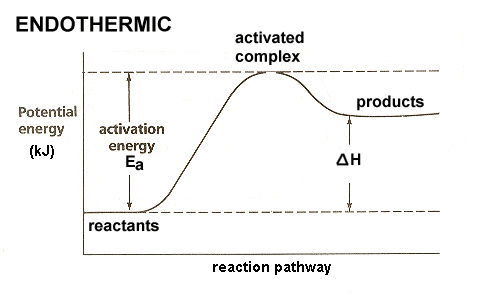
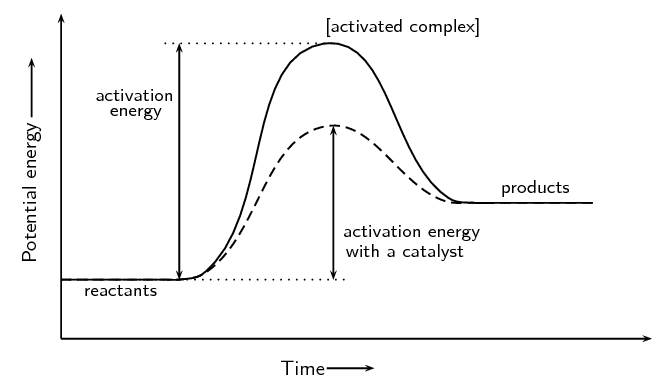
Powered by FlexBook® textbook Platform ® © CK-12 Foundation 2021; Please wait... Please wait... 09-07-2021 · A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left ... An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. Exothermic ...
Exothermic potential energy diagram. As shown in the energy diagram, the hydrogenation of alkenes is exothermic, and heat is released corresponding to the ΔE (colored green) in the diagram. This heat of reaction can be used to evaluate the thermodynamic stability of alkenes having different numbers of alkyl substituents on the double bond. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction. 9 Examples of Chemical Energy. Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction.In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling.
An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. Exothermic ... 09-07-2021 · A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left ... Powered by FlexBook® textbook Platform ® © CK-12 Foundation 2021; Please wait... Please wait...
























0 Response to "40 exothermic potential energy diagram"
Post a Comment