42 in the diagram which part is the anode
In general, common anode displays are more popular as many logic circuits can sink more current than they can source. Also note that a common cathode display is not a direct replacement in a circuit for a common anode display and vice versa, as it is the same as connecting the LEDs in reverse, and... The anode is the positively electrode through which the electrons leave the chemical device. Anode rod 9003892005 attracts and neutralizes...
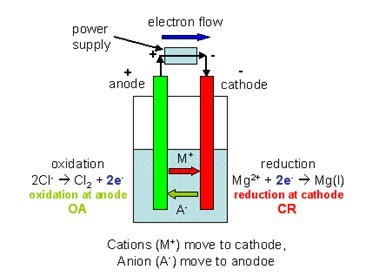
15 Electroplating The anode (the less active metal) is oxidized by the power source. The electrons reduce copper ions in the solution, which coat the cathode. 16 The diagram represents the electroplating of a metal fork with Ag(s). Which part of the electroplating is represented by the fork...
In the diagram which part is the anode
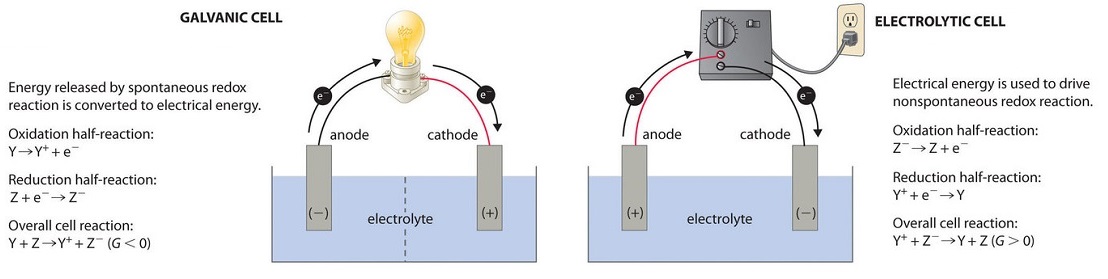
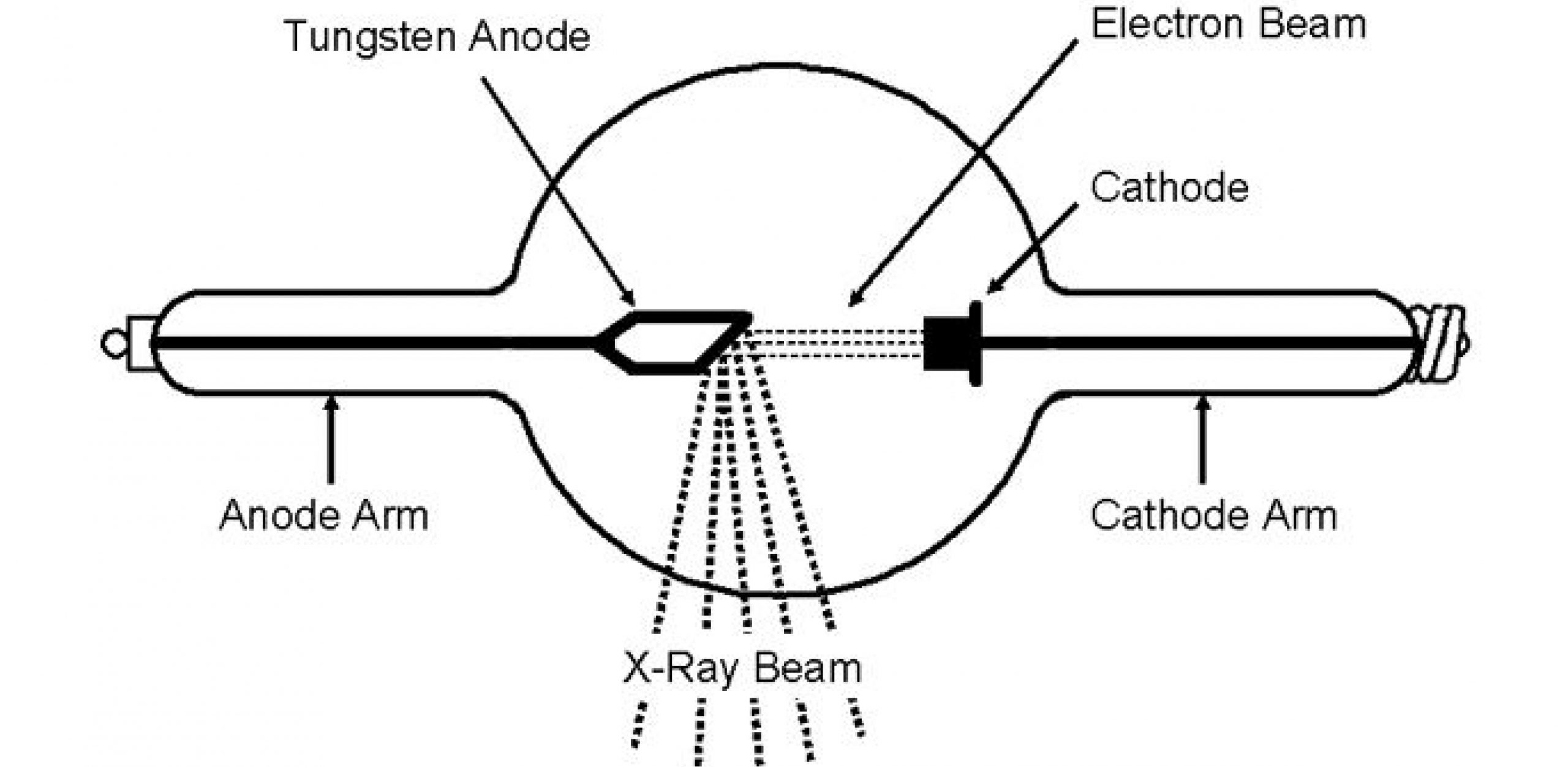
In this diagram, the high speed particle is coming from left to right in a slightly downward trajectory and you will notice that the x-ray struck one of the The target of the electron stream is a small spot on the "anode" part of the x-ray tube. Modern x-ray tubes have an anode disc which you can see in the... Remember that spontaneity is indicated by the change in Gibbs free energy, ΔG. All three types contain electrodes where oxidation and reduction take place. For all electrochemical cells, the electrode where oxidation occurs is called the anode, and the electrode where reduction occurs is called the cathode. That means the anode is connected to the p side and the cathode is connected to the n side. We can create a simple PN junction diode by doping pentavalent or donor impurity in one portion and trivalent or acceptor impurity in the other portion of silicon or germanium crystal block.
In the diagram which part is the anode. Each project circuit diagram, code and detail description is given in the post. All the project's source First configuration has all the led's anode's connected together and this configuration 7 segment display is known as In then the four seven segment display is a dot which sets a part the hour and minute. The diagram shows an aluminium oxide electrolysis cell. Both the negative electrode (cathode) and positive electrode (anode) are made of graphite, which is a form of carbon. Aluminium ions receive electrons at the negative electrode and are reduced to aluminium atoms The cell diagram is a representation of the overall reaction in the electrochemical cell. The chemicals involved are what are actually reacting during the reduction The cell diagram makes it easier to see what is being oxidized and what is being reduced. These are the reactions that create the cell potential. Now, if once we learn to recognize the K, if the diode orientate to a different direction in the diagram, we could easily identify the anode and cathode. In that selected portion's counterpart, or outer-portion of the path, current flows from Anode(of the selected part, here diode) to Cathode ( of the...
Jul 30, 2012 · On the physical LED, the longer lead (or leg) of the LED is the anode. The cathode is marked on the rim of the LED body with a flat area shown in the diagram. Another way to tell which lead is the anode and which is the cathode is to look at the two plates at the end of the leads inside the body of the LED. The bigger plate will be the cathode. if H+ is part of the electrode reaction, then under dilute approximation, aH+ ∼ cH+. The pH of the electrolyte is dened as the negative of the natural As shown in the Pourbaix diagram of these Fig2, if we impose a potential on the anode higher than. E1, or the potential on the cathode lower than E2... Start studying X-Ray Tube ANODE (part 1). Learn vocabulary, terms and more with flashcards, games and other study tools. What is the function of the anode? Serves as a target surface for the high voltage electrons from the filament, conducts the high voltage from the cathode back into the x-ray... Anode rod water heater accessories water heater parts the home depot. For your suburban sw6de you will want the camco water heater element cam02143 and Cutaway diagram of a triode vacuum tube showing the plate anode in electronic vacuum devices such as a cathode ray tube the anode is the...
An anode is an electrode through which the conventional current enters into a polarized electrical device. In an electrolytic cell, the anode is the wire or plate having excess positive charge.[2] Consequently, anions will tend to move towards the anode where they can undergo oxidation. Cell diagrams for galvanic cells or voltaic cells tutorial with worked example for the Daniell Cell for chemistry students. The anode is shown on the left hand side of the diagram (that is, the oxidation reaction is shown on the left) . An anode is an electrode through which the conventional current enters into a polarized electrical device. This contrasts with a cathode, an electrode through which conventional current leaves an electrical device. A common mnemonic is ACID, for "anode current into device". (4) (b) The anode is at a positive potential of 4200 V with respect to the filament. (i) Calculate the kinetic energy, in J, of an electron in the beam in part In an experiment to measure the charge on a charged oil droplet, a droplet was observed between two horizontal metal plates, as shown in the diagram...
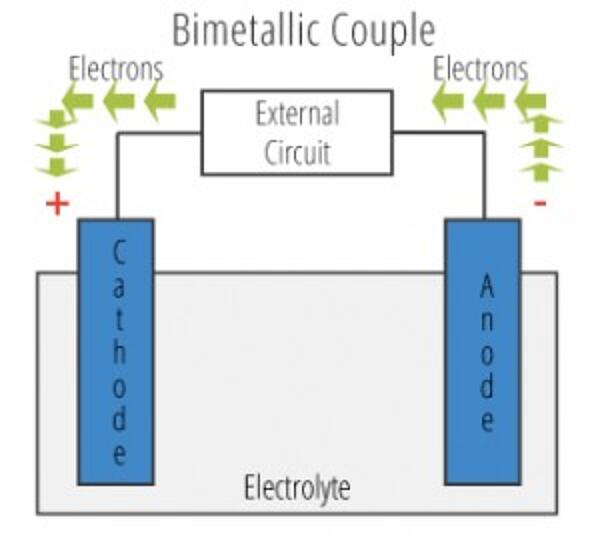
The anode and cathode are defined by the flow of current. In the general sense, current refers to any movement of electrical charge. However, you should keep in mind the convention that current direction is according to where a positive charge would move, not a negative charge. So, if electrons do the...
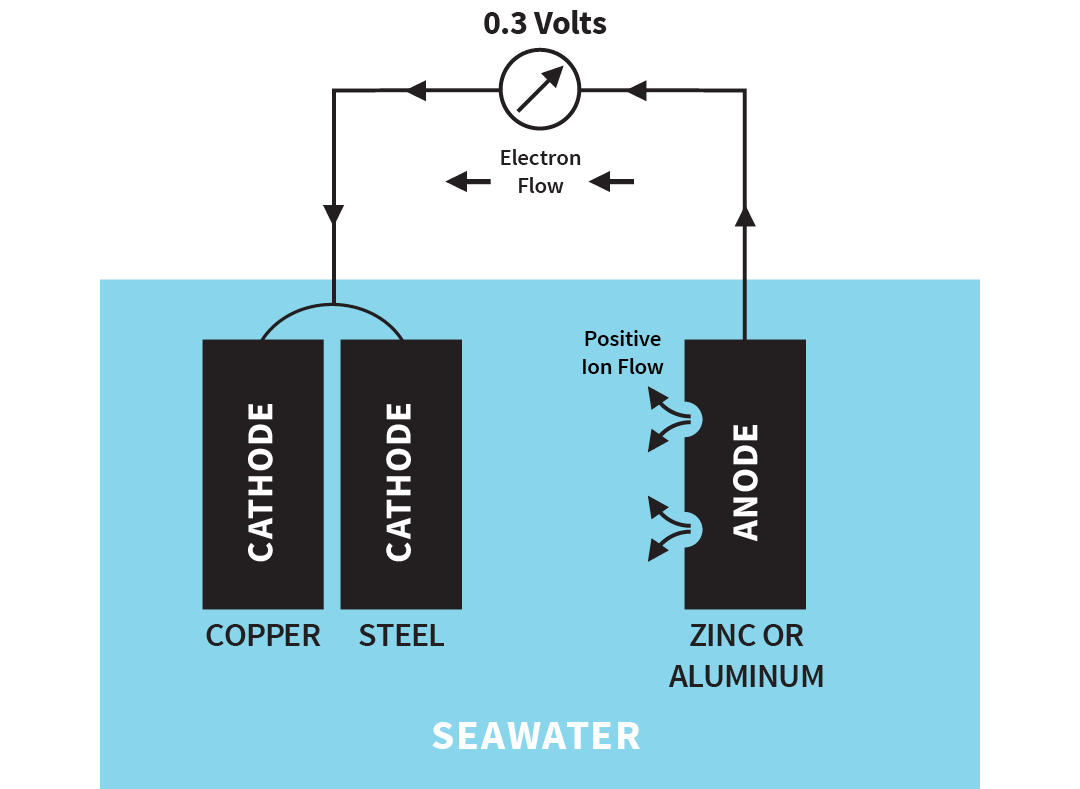
The anode part of the surface is corroded. In the case of low pH the cathode reaction will be: 2e- + 2H+ → H2. The cathode surface may be an impurity, such as an The electropotentials of metals can be measured in different water solutions and listed in galvanic series, as for seawater in the diagram.
Anode and Cathode are the two classifications in which electrodes are classified. People generally in misconception regards anode particularly as positive and cathode particularly as negative.
To be able to determine the anode and cathode, one must know about the chemistry of the reaction in the cell. Cells with different kinds of I hope the explanations below are readable, since it was best done with a hand-drawn diagram rather than the capabilities of the limited text processing in Quora…
Diagram of a zinc anode in a galvanic cell. Note how electrons move out of the cell, and the conventional current moves into it in the opposite direction. In a battery or galvanic cell, the anode is the negative electrode from which electrons flow out towards the external part of the circuit.
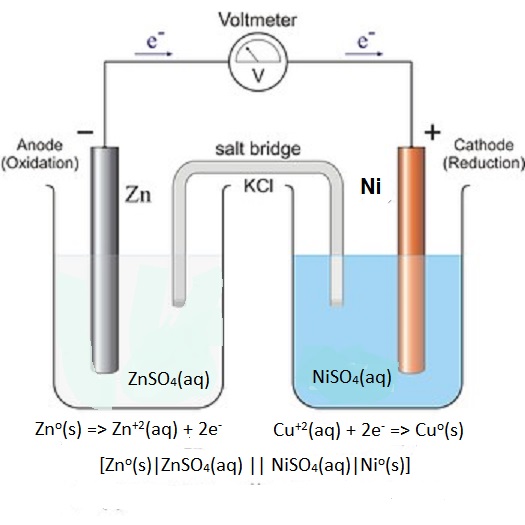
Apr 21, 2019 · Anode is the electrode (metal) at which oxidation process is occurred (electrons produced). Cathode is the electrode (metal) at which reduction process is occurred (electrons consumed). In the shown diagram: Zn is oxidized to Zn²⁺ and produces 2 electrons. Cu²⁺ is reduced to Cu and consumes 2 electrons. So, the anode is solid Zn.
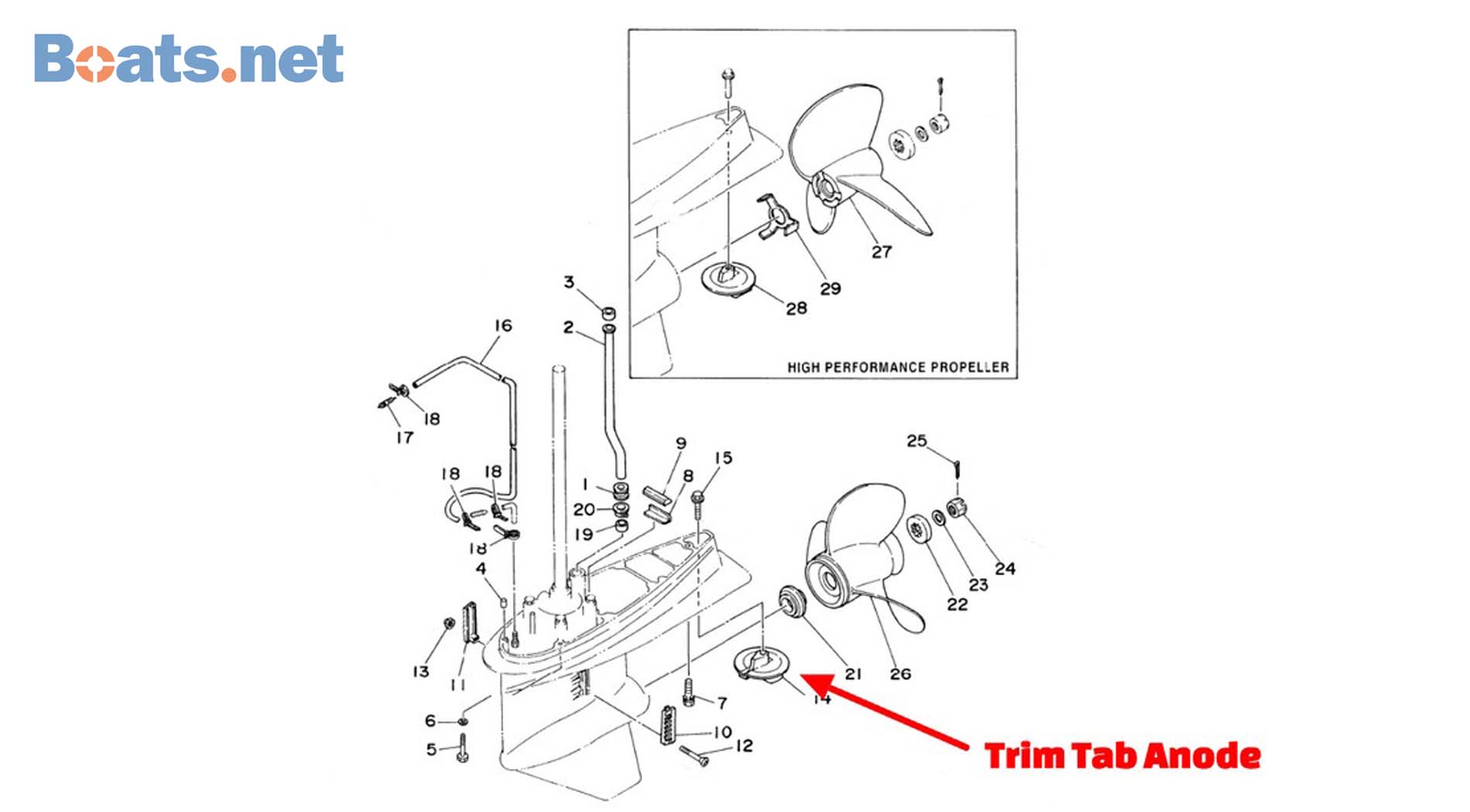
The basics of electric cells. 821631a mercruiser alpha one block aluminum anode 55989a mercurymercruiser nut aluminum anode qty 2 alpha one...
Diagram of a zinc anode in a galvanic cell. Note how electrons move out of the cell, and the conventional current moves into it in the opposite direction. An anode is an electrode through which conventional current (positive charge) flows into the device from the external circuit, while a cathode is...
May 27, 2016 · ROTATING ANODE. The rotating anode is disk shaped and rotates on an axis through the center of the tube (Fig. 2-7). The disk is approximately 3 inches in diameter with a beveled edge. It is composed of tungsten or some similar alloy that can withstand high temperatures. The spindle on which the anode is mounted usually is made of molybdenum.
Which electrode is the anode and which is the cathode? Results. During the experiment, you should have noticed the following It was the Italian physician and anatomist Luigi Galvani who marked the birth of electrochemistry by making a link between chemical reactions and electricity.
Diagram of a zinc anode in a galvanic cell. Note how electrons move out of the cell, and the conventional current moves into it in the opposite direction. In a battery or galvanic cell, the anode is the negative electrode from which electrons flow out towards the external part of the circuit.
The anode characteristics are the most useful set of curves for a valve, and the plot shows anode current Ia against anode voltage Va, for differing An example VHDL code design for this arrangement can be seen with reference to the block diagram shown in Figure 8.54 . This shows a pictorial...
One part sums up how the cell reacts to the anode and the other to the cathode. At the anode , the solid zinc metal transforms into zinc ions due to the oxidation process while it releases 2 electrons. It allows the movement of anions towards anodic and cations towards cathodic sections.
Using this equation: Ecell=Ecathode-Eanode When we see two ions/ elements without an chemical equation, can we determine which element is a the anode and which is at the cathode by looking at their standard E?
Of course, the electrons leave from the negative terminal. Therefore, the answer is that the negative (-) electrode can be defined as the anode, whereas the positive (+) electrode can be given as cathode. If there are any arrows given in the diagram, those represent the direction of electron flow.
This cell diagram does not include a double vertical line representing a salt bridge because there is no salt bridge providing a junction between two dissimilar solutions. Moreover, solution concentrations have not been specified, so they are not included in the cell diagram.
That means the anode is connected to the p side and the cathode is connected to the n side. We can create a simple PN junction diode by doping pentavalent or donor impurity in one portion and trivalent or acceptor impurity in the other portion of silicon or germanium crystal block.
Remember that spontaneity is indicated by the change in Gibbs free energy, ΔG. All three types contain electrodes where oxidation and reduction take place. For all electrochemical cells, the electrode where oxidation occurs is called the anode, and the electrode where reduction occurs is called the cathode.
In this diagram, the high speed particle is coming from left to right in a slightly downward trajectory and you will notice that the x-ray struck one of the The target of the electron stream is a small spot on the "anode" part of the x-ray tube. Modern x-ray tubes have an anode disc which you can see in the...














:max_bytes(150000):strip_icc()/daniell-cell-bdd32b7d694445f5ba3d35e47167e734.jpg)



:max_bytes(150000):strip_icc()/GettyImages-10943484541-1145b5ddea574a1ab5d32aa398291feb.jpg)






0 Response to "42 in the diagram which part is the anode"
Post a Comment