40 lih molecular orbital diagram
GitHub - tomdbar/naqs-for-quantum-chemistry: Supporting ... (In practice these are anti-symetterised tensor products of single-electron orbitals, but for our purposes let's not worry about the details.) Typically, the number of Slater determinants - and so the complexity of optimisation - grows exponentially with the system size, but recently machine learning (ML) has emerged as a possible tool with ... Molecular Orbital Theory: Explanation, Illustrations and ... The molecular orbital energy level diagram for lithium is shown below.
The GW Miracle in Many-Body Perturbation Theory for the ... where the factor of 2 accounts for spin, p and q are molecular orbital (MO) indices, ϵ p and ± iη is a vanishing imaginary number that ensures the correct analytic behavior of G 0. G 0 is diagonal in the corresponding MO basis. In practice, we make the further approximation that the self-energy difference is also diagonal in the MO basis:
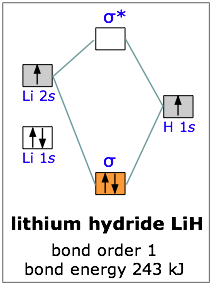
Lih molecular orbital diagram
The dipole moment of LiH is ` 1.964xx10^(-29)` Cm and the ... The dipole moment of LiH is ` 1.964xx10^ (-29)` Cm and the interatomic distance between Li and H in the molecule is ` 1 .596Å` . Calualate the persent ionic character of the molecule . Updated On: 8-12-2021. This browser does not support the video element. (PDF) Many-particle covalency, ionicity, and atomicity on ... PDF | We analyze two-particle binding factors of H2, LiH, and HeH+ molecules/ions with the help of our original exact diagonalization ab intio (EDABI)... | Find, read and cite all the research you ... diagramweb.net › molecular-orbital-diagram-of-lihMolecular Orbital Diagram Of Lih Feb 28, 2019 · Molecular Orbital Diagram Of Lih. and 2p orbitals, but that is not how sodium chloride is made. Sodium atoms are Construct an MO diagram for LiH and suggest what type of bond it might have. Nonbonding sigma is occupied, and then the sigma orbital is occupied. Net effect : Li-H forms a stable bond. LiH MO diagram. You might have.
Lih molecular orbital diagram. A unified molecular‐wide and electron density based ... Polyamines are extremely flexible molecules and often unexpected BPs are found in equilibrium structures. 22 Molecular graphs of protonated triethylenetetramine ( trien, HL) of (i) the lowest energy conformer (LEC; HL-1) and (ii) the second lowest energy HL-2 (with Δ E = EHL-2 − EHL-1 = 0.70 kcal/mol) found at the MP2 level are shown in Figure 1. programming - Ground state compute issue by qiskit ... 1 I use the following code to calculate the ground state for the LiH molecule in an active space. I come across two problems. The first is I found that the Hartree Fock state gave energy that is far from the ground state energy. But the qc state is the true Hartree Fock state. The second problem is using the ground_state, it just outputs 0. H2S Lewis Structure, Molecular Geometry, Hybridization and ... H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity. Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H2S. The molecule has two Hydrogen atoms and a single Sulfur atom. H2S is also a precursor for elemental Sulfur. Molecules | Free Full-Text | The Valence-Bond (VB) Model ... (i = 1,4) orbitals are specified, the wave function (6) could represent any four-electrons system, for example, the Be atom or the LiH molecule. What all the four-electron systems have in common is the fact that their wave functions must be basis for the S4 group, once the permutation symmetry of the Hamiltonian is properly taken into account.
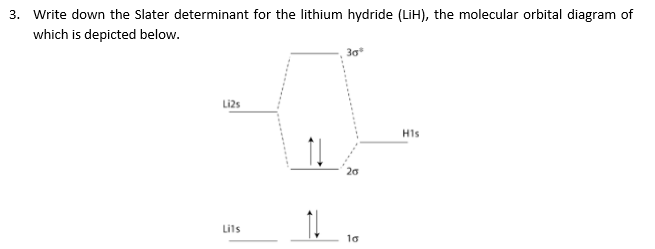
schematron.org › molecular-orbital-diagram-of-lihMolecular Orbital Diagram Of Lih - schematron.org Jul 05, 2019 · We shall consider the molecular orbitals in LiH, CH and HF to illustrate how molecular orbital theory describes the bonding in heteronuclear molecules, and to.The molecular orbital energy level diagram of LiH in conventional textbooks for quantum chemistry is incorrect from viewpoint of ab initio Hartree-Fock SCF-MO calculation, because the 2σlevel of LiH is drawn at a lower position than the 1s orbital of H. › homework-help › questions-andSolved 3. Write down the Slater determinant for the lithium ... Transcribed image text: 3. Write down the Slater determinant for the lithium hydride (LiH), the molecular orbital diagram of which is depicted below. Li2s H1s 20 Lils lơ Write down the Slater determinant for Be (1s22s2) and the first excited state of Li. The ground state of Li is 1s 2s1 4. 9.15: Molecular Term Symbols Designate Symmetry ... The molecular orbital diagram for \(O_2\) is. Where I chose arbitrary configurations for the last two electrons. There are two open ‐ shell electrons occupying the anti‐bonding \(π_g\) orbitals. These are the only electrons that matter. It is easiest to simply draw all of the permutations and figure out the bounds on \(Λ\) and \(M_L\) by ... Supported Ï â Complexes of Liâ C Bonds from Coordination ... 4 as a source of LiH. Contradicting our expectations, instead of forming the anticipated Mo C Li H Mo metallacyclic rings, LiH promoted Mo CH 3 to Mo H bond metathesis[34] and elimination of LiCH 3, ultimately generating a hydride-rich Mo 6 Li 9 H 18 cluster, recently prepared by our group by a different procedure.[35] Results and Discussion
Linear-scaling explicitly correlated treatment of solids ... Theory and implementation of the periodic local MP2-F12 method in the 3*A fixed-amplitude ansatz is presented. The method is formulated in the direct space, employing local representation for the occupied, virtual, and auxiliary orbitals in the form of Wannier functions (WFs), projected atomic orbitals (PAOs), and atom-centered Gaussian-type orbitals, respectively. PRX Quantum 2, 020337 (2021) - Correlation-Informed ... Encoding strongly entangled spin-orbitals into proximal qubits on a quantum chip naturally reduces the circuit depth needed to prepare the ground state. For representative molecular systems, LiH , H 2 , ( H 2 ) 2 , H 4 ≠ , H 3 + , and N 2 , we demonstrate that placing entangled qubits in close proximity leads to shallower depth circuits ... Diborane - Wikipedia Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B 2 H 6.It is a colorless, pyrophoric gas with a repulsively sweet odor. Synonyms include boroethane, boron hydride, and diboron hexahydride. Diborane is a key boron compound with a variety of applications. Methane - Wikipedia RTECS number PA1490000 UNII OP0UW79H66 Y UN number 1971 CompTox Dashboard(EPA) DTXSID8025545 InChI InChI=1S/CH4/h1H4 Y Key: VNWKTOKETHGBQD-UHFFFAOYSA-N Y SMILES C Properties Chemical formula CH4 Molar mass 16.043 g·mol−1 Appearance Colorless gas Odor Odorless Density 0.657 kg·m−3(gas, 25 °C, 1 atm) 0.717 kg·m−3(gas, 0 °C, 1 atm)[2]
Gate-free state preparation for fast variational quantum ... Molecular modeling stands in the juncture of key advances in many important fields including and not limited to energy storage, material designs, and drug discovery.
Qubit-excitation-based adaptive variational quantum ... We benchmark the performance of the QEB-ADAPT-VQE with classical numerical simulations for small molecules: LiH, H 6, and BeH 2. In the section "Energy dissociation curves", we compare the...
Space-warp coordinate transformation for efficient ionic ... where ψ a,l and ψ b,m are primitive GTOs, their indices l and m refer to different orbitals centered on atoms a and b, and i and j are coordinates of spin-up and spin-down electrons, respectively. When the JAGPs is expanded over p = N el /2 molecular orbitals, the JAGPs coincides with the Jastrow-Slater determinant (JSD) Ansatz. 5,57 5. F.
Molecules | Free Full-Text | Perturbing the O-H ... - MDPI Ab initio MP2/aug'-cc-pVTZ calculations have been carried out to identify and characterize equilibrium structures and transition structures on the 1-oxo-3-hydroxy-2-propene: Lewis acid potential energy surfaces, with the acids LiH, LiF, BeH2, and BeF2. Two equilibrium structures, one with the acid interacting with the C=O group and the other with the interaction occurring at the O-H group ...
› article › jccjA Molecular Orbital Energy Level Diagram of LiH We show an molecular orbital energy level diagram of LiH obtained by ab initio Hartree-Fock SCF-MO calculation with 6-311++G** basis set in this note. The 2σlevel of LiH is drawn at a higher position than the 1s of H in this diagram. The 1s electron of H is thus destabilized in LiH. Since the 2s electron of Li comes close to the H atom, the 1s electrons of H in 2σorbital are destabilized by electron repulsion.
Quantum computing hardware in the cloud: - arXiv Vanity To date, the most advanced VQE simulations have mapped just 24 molecular orbitals onto 12 qubits , a relatively easy feat for traditional computers. In order to calculate the energy ground state of more complex systems with chemical accuracy, it is expected that the number of qubits available will need to increase by orders of magnitude.
(PDF) Molecular excited state VQE simulations with ... Energy convergence plots for the first excited states of LiH and BeH 2 in the STO-3G basis at equilibrium bond distances of r Li-H = 1.546Å546Å and r Be-H = 1.316Å316Å, respectively.
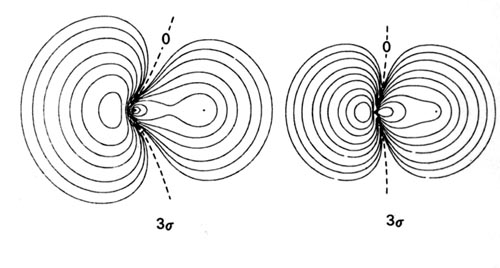
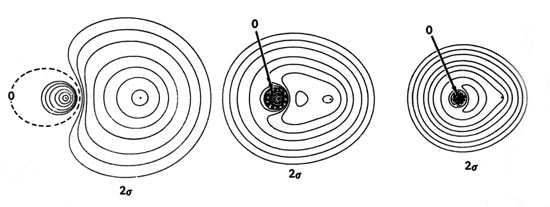
(Get Answer) - Consider the two terminal molecular device ... Images of molecular orbitals for LiH calculated using the minimal basis set are shown below. In these images, the smaller atom is H. The H 1s AO has a lower energy than the Li 2s AO. The energy of the MOs is (left to right) -63.9 eV, -7.92 eV, and...
Variational quantum eigensolver simulations with the ... For the LiH molecule with R (Li-H) = 1.0, 2.0, 3.0, and 4.0 Å, we employed the STO-3G basis set and performed 12 qubit simulations. The geometry of the P4 cluster is illustrated in Fig. 3. The P4 cluster consists of two parallel hydrogen molecules with nuclei in a rectangular arrangement.
9.8: Molecular Orbital Theory - Chemistry LibreTexts The lithium 1 s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
Augmented Lagrangian method for spin‐coupled wave function ... In this study, we validated feasibility of the derivative-free augmented Lagrangian method for optimizing the spin-coupling and the orbital coefficients with the constraint of normality of the wave function. We employed this SCGVB method to compute dissociative potential energy curves (PECs) of H 2, , , and LiH. The obtained PECs by the SCGVB ...
Suppressing Competitive Coordination Reaction for Ohmic ... Undoubtedly, we observed that the dihedral angles between the metal-coordinated phen-ring and the lone-pair electrons plane for p-Pip-phen and p-Pyr-phen were 42.5° and 26.5°, respectively, both smaller than that of p-Amn-phen (48.3°), indicating that the exocyclic nitrogen p orbitals of p-Pip-phen and p-Pyr-phen assumed a more coplanar ...
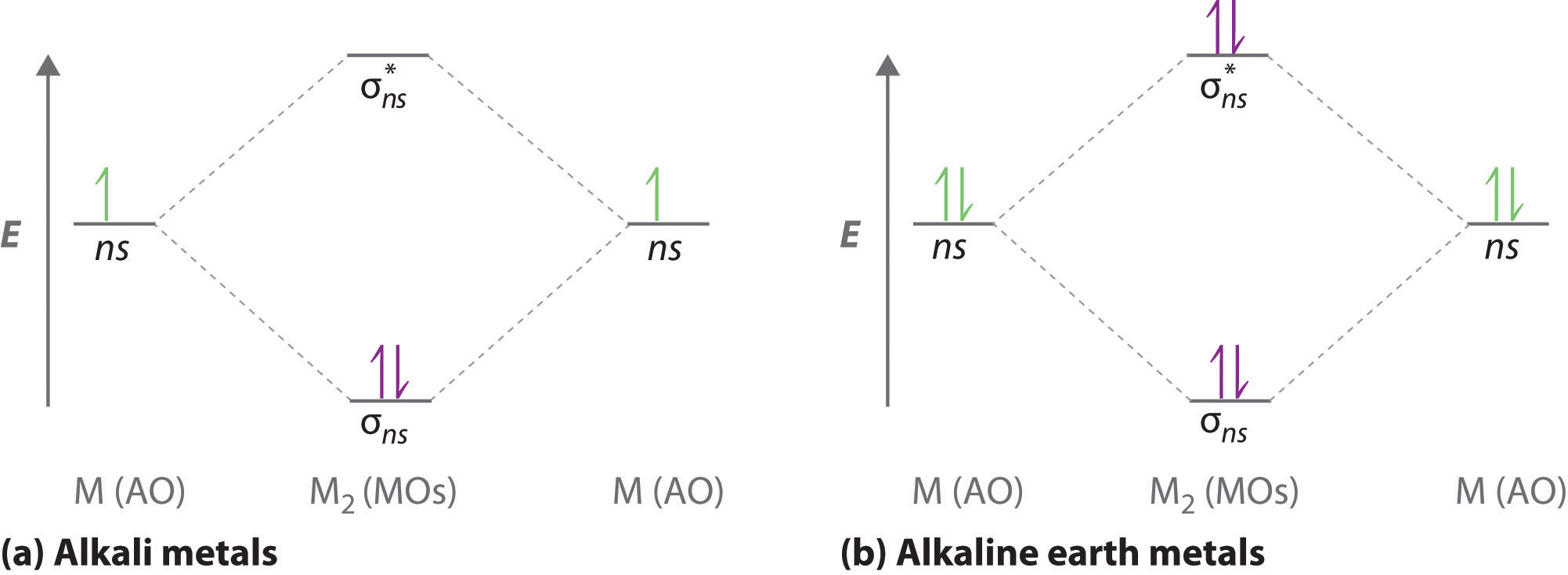
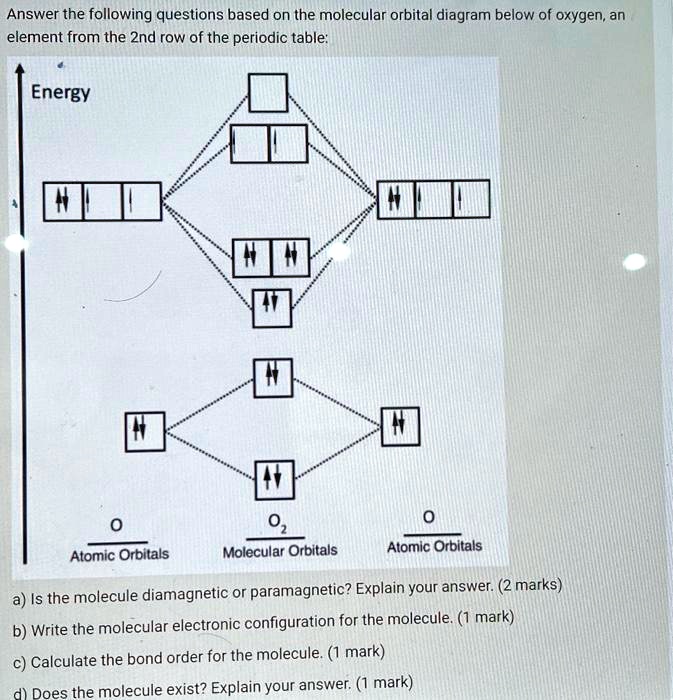
Maharashtra Board Class 11 Chemistry Important Questions ... The π molecular orbitals has a node between the nuclei. Diagram: Question 56. Write the increasing order of energies of molecular orbitals in various diatomic molecules of second row elements. Answer: The increasing order of energies of molecular orbitals for molecules (except O 2 and F 2) is:
Active space chosen for VQE in qiskit - Quantum Computing ... Under the "Running VQE on a Statevector Simulator" part, the code provided for leveraging active space of LiH to reduce the qubit requirement seems quite confusing. The freeze_list in the code is interpreted as the core space in my opinion. However, what confuse me is that why to choose the [-3, -2] orbitals as virtual space to be removed ...
VARIATIONAL QUANTUM EIGENSOLVER FOR "LiH" MOLECULE by ... The LiH has axial symmetry, H has 1s orbital, and Li has 1s, 2s, and px, py, pz orbitals. 6 in total. Li 1s has core electrons that shouldn't form the bond. The ground state of this molecule is a...
diagramweb.net › molecular-orbital-diagram-of-lihMolecular Orbital Diagram Of Lih Feb 28, 2019 · Molecular Orbital Diagram Of Lih. and 2p orbitals, but that is not how sodium chloride is made. Sodium atoms are Construct an MO diagram for LiH and suggest what type of bond it might have. Nonbonding sigma is occupied, and then the sigma orbital is occupied. Net effect : Li-H forms a stable bond. LiH MO diagram. You might have.
(PDF) Many-particle covalency, ionicity, and atomicity on ... PDF | We analyze two-particle binding factors of H2, LiH, and HeH+ molecules/ions with the help of our original exact diagonalization ab intio (EDABI)... | Find, read and cite all the research you ...
The dipole moment of LiH is ` 1.964xx10^(-29)` Cm and the ... The dipole moment of LiH is ` 1.964xx10^ (-29)` Cm and the interatomic distance between Li and H in the molecule is ` 1 .596Å` . Calualate the persent ionic character of the molecule . Updated On: 8-12-2021. This browser does not support the video element.
![Solved] Images of molecular orbitals for LiH calculated using ...](https://s3.amazonaws.com/si.question.images/images/question_images/1525/4/9/6/3235aed3a03c86c71525496315701.jpg)





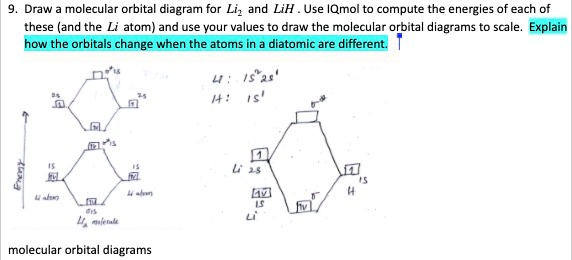














0 Response to "40 lih molecular orbital diagram"
Post a Comment